주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| Title | How a Natural Compound from Sea Squirts Combats Cancer | ||
|---|---|---|---|
| Embargo date | 2024-03-13 10:18 | Hits | 209 |
| Press release |
![docx 파일명 : [v7] KS_Kook et al_Press Release-20240221.docx](/images/mimetype/docx.gif) [v7] KS_Kook et al_Press Release-20240221.docx
[v7] KS_Kook et al_Press Release-20240221.docx
|
||
| att. | |||
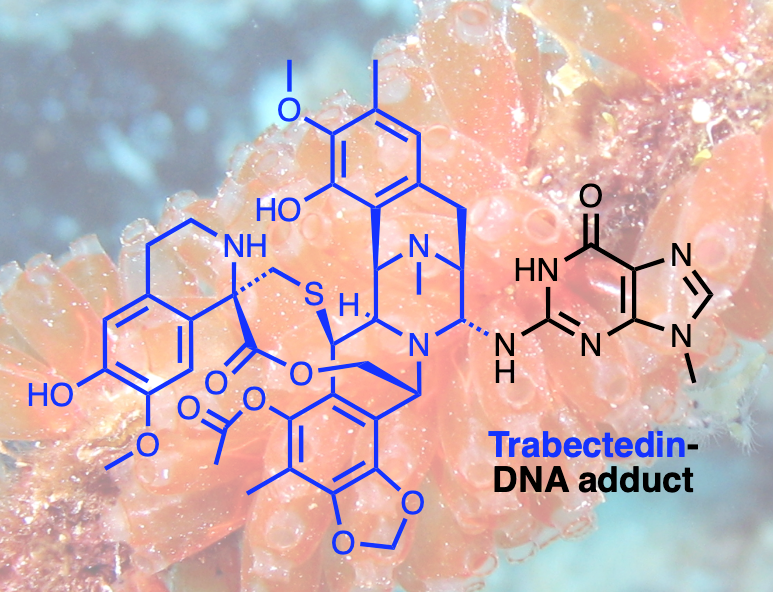
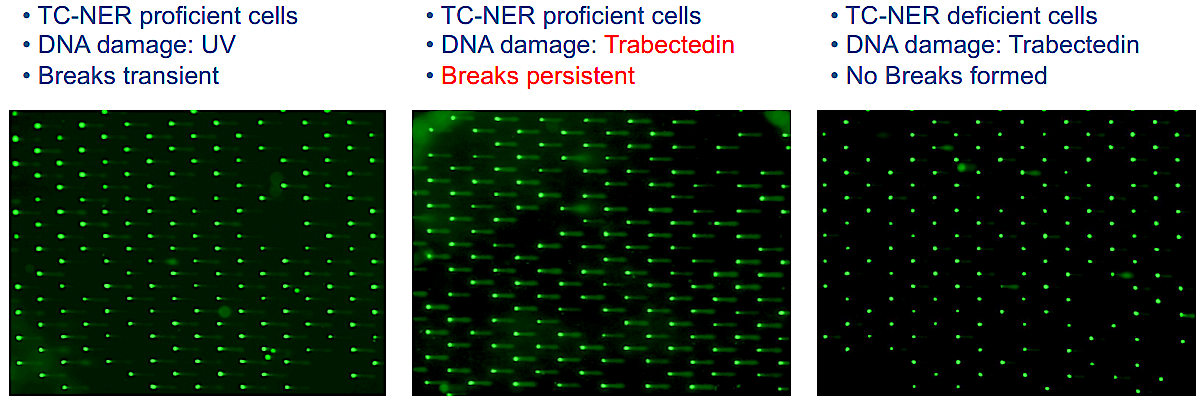
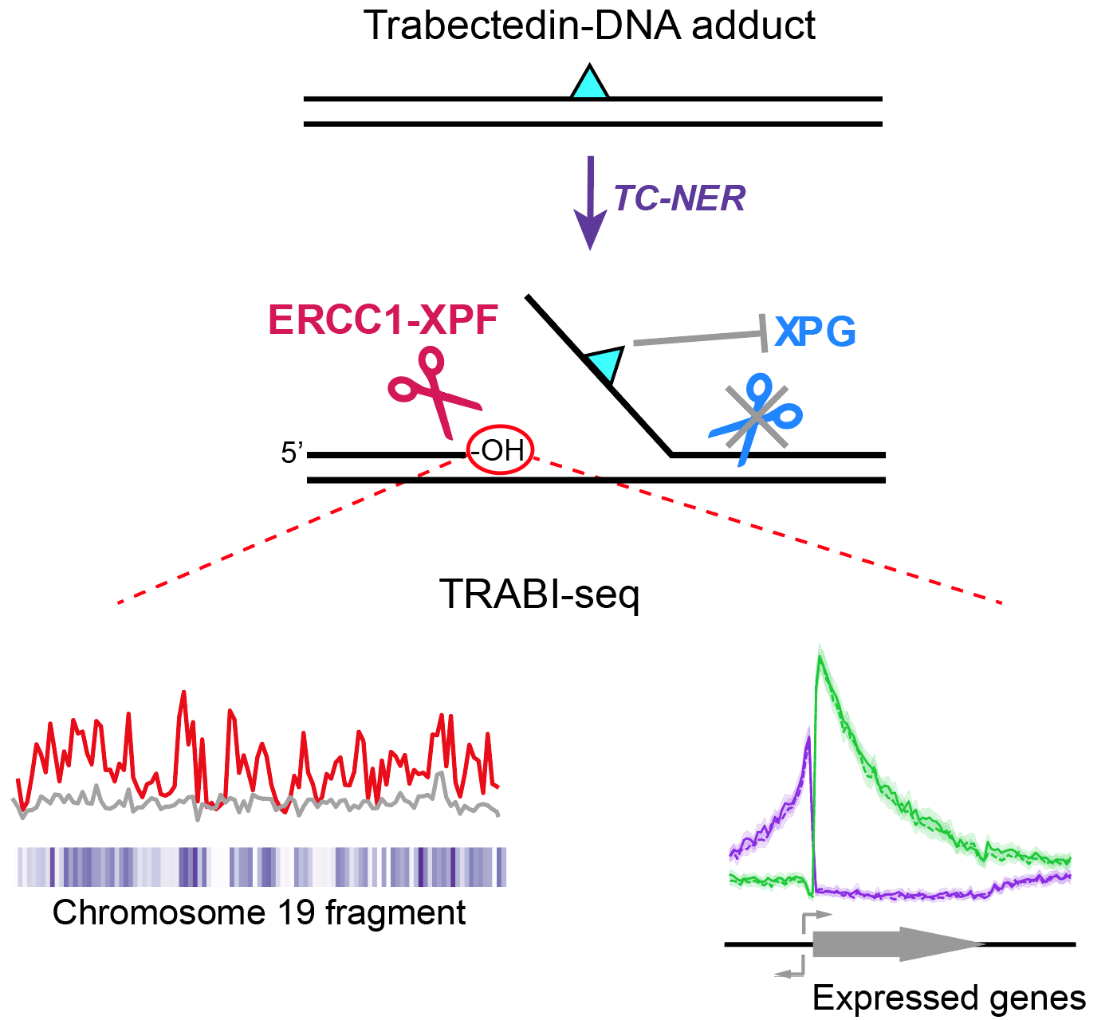
How a Natural Compound from Sea Squirts Combats Cancer- Scientists have deciphered how trabectedin outsmarts cancer cells’ DNA repair mechanism, paving the way for tailored treatments - Numerous anti-cancer drugs function by targeting the DNA within cancerous cells, halting their proliferation. Yet, cancer cells occasionally develop mechanisms to repair the damage inflicted by these drugs, diminishing their effectiveness. Consequently, physicians are increasingly embracing a novel approach to cancer treatment known as precision medicine. This method involves selecting medications that precisely align with the unique attributes of an individual's cancer. Precision medicine proves particularly beneficial in addressing cancers that have evolved to evade conventional treatments. Trabectedin, a promising drug derived from the sea squirt Ecteinascidia turbinata, has shown potential in combating cancers resistant to conventional treatments. However, its precise mechanism of action has remained elusive—until now. A collaborative effort led by Dr. SON Kook and Professor Orlando D. SCHÄRER from the Center for Genomic Integrity within the Institute for Basic Science in South Korea, along with Dr. Vakil TAKHAVEEV and Professor Shana STURLA from ETH Zurich, Switzerland, has illuminated the inner workings of this mysterious compound. Using highly sensitive high throughput COMET Chip assays to detect breaks formed in the genomes of cells, IBS researchers revealed trabectedin induces persistent breaks in the DNA of cancer cells. The researchers showed that these DNA breaks are only formed in cells with high levels of DNA repair, specifically those that operate a pathway called transcription-coupled nucleotide excision repair (TC-NER). TC-NER is a vital mechanism that identifies DNA damage during transcription, initiating repair processes involving two endonucleases ERCC1-XPF and XPG. Trabectedin’s DNA damage disrupts this process by allowing the initial incision by ERCC1-XPF but blocking the subsequent action by XPG, halting the TC-NER process. This disruption of the repair process leads to long-lasting DNA breaks that ultimately kill cancer cells. Analysis of the DNA break patterns induced by trabectedin revealed that breaks are formed throughout the genome, but only at sites where active transcription and with it, TC-NER occurs. Using this new insight into the mechanism of how DNA breaks are accumulated, the researchers sought to determine where in the genome these breaks occur. This led to the development of a new method called TRABI-Seq (for TRABectedin-Induced break sequencing), which allows for the precise identification of trabectedin's action sites within tumor cell DNA. “This incision by ERCC1-XPF creates a markable free hydroxyl group in the DNA, enabling us to sequence DNA and locate these breaks,” explains Dr. Son. TRABI-Seq is being tested on various cancer cells to determine trabectedin's efficacy in targeting tumors with advanced DNA repair capabilities, often associated with elevated transcription levels due to oncogene activation. It is hoped that these findings will help position trabectedin as both a predictive marker to identify vulnerable cancers and a therapeutic option for precision treatment. With its ability to target tumors resistant to conventional therapies, trabectedin may provide further hope in the fight against drug-resistant cancer with highly active DNA repair capabilities.
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS)
|
|||
| Next | |
|---|---|
| before |
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20