December 11 – 12, 2025
2F, Grand Ballroom, Daejeon Convention Center 1 (DCC 1),
Daejeon, South Korea
Welcome to the IBS-KAI-KSV Conference on
Infection and Immunity
“IBS-KAI-KSV Conference on Infection and Immunity” aims to bring together professionals and experts from various fields to exchange ideas, insights, and experiences on Infection and Immunity area. With a diverse range of speakers, interactive sessions, and networking opportunities, the conference promises to be both informative and engaging.
Date & Venue
December 11th – 12th, 2025
2F, Grand Ballroom ,Daejeon Convention Center 1 (DCC 1), Daejeon, South Korea
Program
| Time | Program |
|---|---|
| 08:30-09:20 | Registration |
| 09:20-09:30 | Opening Remarks |
| Session 1. Emerging and Re-emerging Viruses I / Chairs: Richard Webby, Nam-Hyuk Cho | |
| 09:30-10:10 | Peiris, Joseph Sriyal Malik (The University of Hong Kong, China) MERS coronavirus: A pandemic threat? |
| 10:10-10:40 | Atsushi Kawaguchi (University of Tsukuba, Japan) Molecular basis of SARS-CoV-2 multiorgan infection via viremia |
| 10:40-11:10 | Young Ki Choi (Korea Virus Research Institute, IBS, Korea) Animal organoid model systems and their applications for studying of emerging and re-emerging viruses |
| 11:10-11:40 | SangJoon Lee (Ulsan National Institute of Science and Technology, Korea) AIM2 Inflammasome as a Double-Edged Sword in Viral Immunity |
| 11:40-13:00 | Lunch |
| Session 2. Emerging and Re-emerging Viruses II / Chairs: Peiris, Joseph Sriyal Malik, Jae Ung Jung | |
| 13:00-13:40 | Richard Webby (St. Jude Children’s Research Hospital, USA) A(H5N1) influenza viruses in the Americas |
| 13:40-14:10 | Min-Suk Song (Chungbuk National University, Korea) A SARS-CoV-2 Mpro mutation conferring ensitrelvir resistance paradoxically increases nirmatrelvir susceptibility |
| 14:10-14:40 | Yong Taik Lim (Sungkyunkwan University, Korea) Immune Designer Nano-vaccines for Reshaping Dynamic Immunity |
| 14:40-15:10 | Rajendra Karki (Seoul National University, Korea) Innate Immune Recognition of DAMPs and PAMPs in Liver Disease Pathogenesis |
| 15:10-15:40 | Break |
| Session 3. Emerging and Re-emerging Viruses III / Chairs: Jin-Hyun Ahn, Moon Jung Song | |
| 15:40-16:20 | Jae Ung Jung (Cleveland Clinic, USA) Inhibiting the Nsp14–Nsp10 interface impairs SARS-CoV-2 replication |
| 16:20-16:50 | Benjamin Brennan (University of Glasgow, UK) SFTSV NSs protein is a novel tick antiviral RNAi response suppressor |
| 16:50-17:20 | Nam-Hyuk Cho (Seoul National University, Korea) Recombinant Bandavirus as an effective bivalent vaccine platform inducing protective immunity against intracellular pathogen and cancer |
| 17:20-17:50 | Jong-Eun Park (Korea Advanced Institute of Science and Technology, Korea) Lifetime dynamics of immune cells and the immune repertoire revealed by integrative single-cell analysis |
| Time | Program |
|---|---|
| Session 4. Host-Virus Interaction / Chairs: Young Ki Choi, Jong-Eun Park | |
| 09:30-10:10 | V. Narry Kim (IBS, Seoul National University, Korea) Exploring the Virosphere to Uncover Hidden Mechanisms |
| 10:10-10:40 | Jin-Hyun Ahn (Sungkyunkwan University, Korea) G-quadruplex regulation of cytomegalovirus infection |
| 10:40-11:10 | Moon Jung Song (Korea University, Korea) The Molecular Gatekeepers: NLRC3 and G-quadruplex Dynamics in Controlling Oncogenic Herpesvirus Latency in B Cells |
| 11:10-11:40 | Heeju Ryu (Sungkyunkwan University, Korea) High-dimensional profiling of virus-specific T cells in virus-associated cancer and infection |
| 11:40-13:00 | Lunch |
| Session 5. Host Immune Responses to Viral Infections I / Chairs: Eui-Cheol Shin, You-Me Kim | |
| 13:00-13:40 | Arash Grakoui (Emory University, USA) Inducible Hepatic-Associated Lymphoid Tissue (iHALT): liver as a surrogate secondary lymphoid organ in hepatotropic viral infection |
| 13:40-14:10 | Seung-Woo Lee (Pohang University of Science and Technology, Korea) Epithelial antigen presentation promotes lung regeneration after influenza virus infection |
| 14:10-14:40 | Min Kyung Jung (Korea Virus Research Institute, IBS, Korea) T cell differentiation landscapes after COVID-19 vaccination |
| 14:40-15:10 | Yeonseok Chung (Seoul National University, Korea) Metabolic Control of Humoral Immunity |
| 15:10-15:40 | Break |
| Session 6. Host Immune Responses to Viral Infections II / Chairs: Seung-Woo Lee, Yeonseok Chung | |
| 15:40-16:10 | Masanori Isogawa (National Institute of Infectious Diseases, Japan) Distinct Localization of Cytotoxic and Cytokine-producing CD8+ T Cells in the Liver during OX40-mediated Virus Inactivation |
| 16:10-16:40 | You-Me Kim (Korea Advanced Institute of Science and Technology, Korea) Latent MCMV Infection and MASH |
| 16:40-17:10 | Bertram Bengsch (University of Freiburg, Germany) The many faces of responding T cells during checkpoint therapy between efficacy and toxicity |
| 17:10-17:40 | Eui-Cheol Shin (Korea Advanced Institute of Science and Technology, Korea) Regulation of IL-15-induced NK-like activation of CD8+ T cells in viral infection |
Invited Speakers
Plenary Speakers
Invited Speakers
Biography
Jae Ung Jung
Professor and Chair
Cleveland Clinic, USA
jungj@ccf.org
Dr. Jae Ung Jung is an internationally recognized leader in virology, immunology, and infectious diseases. He began his pioneering career at Harvard Medical School, where he became the first Korean-born tenured professor at Harvard University and served as Chair of the Tumor Virology Division from 1989 to 2007. In 2007, Dr. Jung joined the Keck School of Medicine at the University of Southern California, where he held the Fletcher Jones Foundation Endowed Chair, served as Distinguished Professor and Chair of the Department of Molecular Microbiology and Immunology, and directed the USC Institute of Emerging Pathogens and Immune Diseases.
Dr. Jung currently serves at the Cleveland Clinic as the Betsy B. deWindt Professor, Associate Chief of Emerging Science and Technology, Chair of the Department of Infection Biology and the Department of Cancer Biology, Director of the Global Center for Pathogen and Human Health Research, and Director of Experimental Therapeutics.
He is internationally renowned for his groundbreaking research on herpesvirus-associated cancers, emerging viral pathogens, antiviral immunity, and the development of vaccines and antiviral therapeutics. Among numerous honors, Dr. Jung received the prestigious 2012 Ho-Am Prize in Medicine. He is a Fellow of the American Academy of Microbiology and the American Association for the Advancement of Science and has received both the Scholar Award from the Leukemia & Lymphoma Society of America and the National Cancer Institute’s Outstanding Investigator Award.
Dr. Jung earned his bachelor’s degree (1982) and master’s degree (1984) from Seoul National University. In 1985, he moved to the United States and completed his Ph.D. in Microbiology at the University of California, Davis in 1989, followed by postdoctoral training at Harvard Medical School, completed in 1992.
Biography
Peiris, Joseph Sriyal Malik
Professor
The University of Hong Kong, China
malik@hku.hk
Malik Peiris graduated from the University of Peradeniya, Sri Lanka in 1972, did his doctoral research in virology at the University of Oxford and served as Lecturer and subsequently Senior Lecturer at the University of Peradeniya, Sri Lanka till 1988. He is currently Professor of Virology at the School of Public Health at The University of Hong Kong. He is a clinical and public health virologist, with an interest in emerging virus disease at the animal-human interface, including vector-borne viruses, influenza and coronaviruses (SARS-CoV, MERS-CoV, SARS-CoV-2). In 2003, he identified the novel coronavirus that caused SARS and contributed to understanding its origins, diagnosis and control. More recently, he works on zoonotic, seasonal and pandemic influenza, MERS and COVID-19 using a One Health approach and is interested in drivers of virus spill-over at the animal human-interface and interventions to reduce spill-over risk. He has been elected as Fellow of the Royal Society of London and as a Foreign Member of the US National Academy of Sciences.
Biography
Richard Webby
Faculty
St. Jude Children’s Research Hospital, USA
richard.webby@stjude.org
Richard Webby is currently a Member of the faculty at St Jude Children’s Research Hospital, Memphis, US. His research field of interest is influenza at the human-animal interface.
He started his professional career as an Assistant Member in the Department of Infectious Diseases at St Jude. He has subsequently risen through the ranks and is a Full Member (Professor equivalent) in the new Department of Host-Microbe Interactions.
Richard is a member of the American Academy of Microbiology and is the Director of the World Health Organization Collaborating Center on Studies on the Ecology of Influenza in Animals. He also Directs the St Jude Center of Excellence in Influenza Research and Response.
He received his B.Sc. and Ph.D. from the University of Otago, New Zealand. He then moved to St Jude to conduct his Postdoctoral Fellowship under the mentorship of Professor Robert Webster.
Biography
V. Narry Kim
Director / Professor
IBS / Seoul National University, Korea
narrykim@snu.ac.kr
Narry Kim is a Professor in the School of Biological Sciences at Seoul National University and a founding Director of the RNA Research Center at Institute for Basic Science (IBS). Kim graduated from Seoul National University in 1992 and received her Ph.D. in 1998 from the University of Oxford where she studied lentiviruses and gene delivery. For postdoctoral training, she joined the Gideon Dreyfuss lab at the University of Pennsylvania to study mRNA surveillance. Kim moved back to Seoul National University in 2001 to set up her own group and has been investigating how genes are regulated at the RNA level. The Kim lab delineated the microRNA pathway, identified key factors including DROSHA, and revealed their action mechanisms and structures. Her group also uncovered the roles of noncanonical RNA tailing such as uridylation and mixed tailing in the control of microRNAs, mRNAs, and viral RNAs. She is a recipient of the L’Oreal-UNESCO Women in Science Award, the Hoam Prize, and the Asan Prize; was elected members of KAS, NAS, EMBO, and the Royal Society; and serves on the editorial boards of Science, Cell, Molecular Cell, GD, and EMBO J.
Biography
Arash Grakoui
Professor
Emory University, USA
arash.grakoui@emory.edu
After completing his undergraduate degrees from Washington University in St. Louis, Dr. Grakoui trained initially in molecular virology studying RNA replication and polyprotein processing of alphaviruses (Sindbis) and flaviviruses (yellow fever and hepatitis C virus) with Dr. Charles M. Rice (2020 recipient of Nobel Prize in Medicine) before pursuing PhD in immunology at Washington University in St. Louis. During his postdoctoral training as a Cancer Research Institute Postdoctoral Fellow at The Rockefeller University, he combined his two areas of interest, virology and immunology, to systematically dissect the role of virus-specific effector lymphocytes during persistent infection.
Dr. Grakoui joined Emory University School of Medicine in the fall of 2004 and is currently a Professor of Medicine with tenure, Division of Infectious Diseases at Emory University in Atlanta, GA. He also holds an appointment as a Core Scientist at the Emory National Primate Research Center, a joint appointment at the Department of Microbiology and Immunology, Investigator at the Emory Center for AIDs Research, and Member of the Cancer Immunology Research Program at Winship Cancer Institute.
The overall goal of his laboratory is to understand the inextricable connectivity between chronic viral infection and the immune system. His laboratory has expertise in molecular virology, immunological approaches and liver biology utilizing in vitro tissue culture, mouse, non-human primate and human models. He is committed to making significant contributions to the understanding of liver disease and to the development and application of immunotherapy aimed at controlling chronic viral replication and end-stage liver disease. To date, he has mentored 4 graduate students, 15 postdoctoral fellows, 4 physician scientists, 9 undergraduates and served on more than 40 thesis committees for graduate students in the PhD program. He served as a Director of Graduate Studies for the Immunology and Molecular Pathogenesis Program at Emory University from 2008-2014 and 2018-2021.
Abstract
Inhibiting the Nsp14–Nsp10 interface impairs SARS-CoV-2 replication
Rong Sun1,3, Jesse A. Coker2, Mi-Jeong Kwak1, Woojin Shin1, Shaun R. Stauffer2,3, Jae U. Jung1,2,3*
1Department of Microbial Sciences in Health, 2Center for Experimental Therapeutics, and 3Global Center for Pathogen and Human Health Research, Cleveland Clinic Research, Cleveland, OH
SARS-CoV-2, the etiologic agent of COVID-19, continues to re-emerge and disproportionately threaten older adults. Despite vaccination, breakthrough infections and variant evolution sustain the need for antivirals with novel mechanisms. We report small-molecule leads that disrupt the essential Nsp14–Nsp10 exonuclease (ExoN) complex by targeting its protein–protein interface, thereby compromising viral proofreading and fitness. A high-throughput screen (~60,000 compounds) using an AlphaScreen® PPI assay yielded inhibitors that advanced through a FRET-based exoribonuclease assay, converging on four hits sharing a common scaffold. Binding to apo Nsp14 was confirmed by differential scanning fluorimetry, surface plasmon resonance, and hydrogen–deuterium exchange mass spectrometry. The compounds selectively blocked Nsp14–Nsp10 interaction in a NanoBiT® live-cell assay and suppressed SARS-CoV-2 replication. Structure–activity relationship development, guided by catalog mining and de novo design, produced leads with on-target antiviral activity, high specificity, and low cytotoxicity. These first-in-class Nsp14–Nsp10 PPI inhibitors establish a tractable route to ExoN-directed therapy and provide a promising prototype for next-generation COVID-19 antivirals.
Abstract
MERS coronavirus: A pandemic threat?
Malik Peiris
School of Public Health, The University of Hong Kong
Zoonotic coronaviruses have caused repeated pandemics over the past 300 years and pose significant future pandemic threats. MERS coronaviruses (MERS-CoV) have caused repeated large outbreaks in humans with chains of multiple human-to-human transmission events. We recently identified human-adaptive amino acid substitution (nsp6 L232F) which is repeatedly emerging in MERS-CoV clade B zoonotic transmission, which increases viral replication competence in human ex-vivo respiratory tissue cultures and in lungs of hDPP4 knockin mice. Although zoonotic MERS cases have apparently declined in the Arabian Peninsula in recent years, key knowledge gaps remain. Is the reason for decline in zoonotic MERS in recent years due to early case detection and improved infection prevention & control, genetic changes in recent clade B viruses or due to cross-reactive population immunity arising SARS-CoV-2 infection or vaccination? Although zoonotic MERS-CoV so far has only been reported in the Arabian Peninsula, the vast majority of MERS-CoV infected dromedary camels are found in East, North and West Africa (>75% of the global dromedary population) and Central Asia, but no zoonotic disease has been reported. There is specific serological and T cell response data suggesting that unsuspected MERS-CoV infection continues to occur in Africa. Thus, pandemic MERS may well emerge outside the Arabian Peninsula, in a setting of low awareness of the threat. MERS-CoV across Africa constitutes a distinct virus clade (clade C) which appears to have lower replication competence in ex vivo cultures of the human respiratory tract and hDPP4 knockin mice. Greater clinical awareness and research on MERS-CoV threats beyond the Arabian Peninsula is critically important.
Abstract
A(H5N1) influenza viruses in the Americas
Richard Webby
Department of Host-Microbe Interactions, St Jude Children’s Research Hospital, Memphis, TN 38105, USA.
With the exception of a brief incursion in 2014-2015, the America’s have remained free of the A/goose/Guangdong/1/96-lineage A(H5) viruses that have ravaged many other regions of the world. This changed in late 2021 when detections of the virus were made in Eastern Canada. The virus spread from these initial detections throughout North, Central, then South America causing mass mortality in wild and domestic birds and also in sea mammals. From a human health perspective, over 1000 zoonotic infections with the A/goose/Guangdong/1/96-lineage A(H5) viruses have been detected globally with a mortality of over 50%. Despite these numbers, decades of circulation in birds, and sporadic detections of mammalian adaptive markers the A(H5) viruses have maintained their avian influenza virus features. Fears of this changing to include more mammalian influenza virus phenotypes were exacerbated with the detection of A(H5N1) viruses in dairy cattle in the U.S. Contrary to these fears, the ability of the bovine-sourced A(H5N1) viruses to bind to human virus receptors, transmit between ferrets, and evolve antigenically, all markers associated with elevated risk to humans, have not changed even with more than a year of continued circulation in dairy cows. The occasional spillover of virus from birds to other mammals has similarly lead to limited evidence for mammalian adaptations. While these data are reassuring from a public health perspective, the continued evolution of the A(H5) viruses requires constant assessments and strong collaboration between One-Health partners.
Abstract
Exploring the Virosphere to Uncover Hidden Mechanisms
Narry Kim1,2
1Center for RNA Research, Institute for Basic Science, Seoul 08826, Republic of Korea
2School of Biological Sciences, Seoul National University, Seoul 08826, Republic of Korea
RNAs of external origin, such as viral RNAs and therapeutic mRNAs, rely on cellular machinery for entry and translation while facing cellular barriers that restrict their functions. Thus, for developing effective antivirals and RNA therapeutics, it is important to understand how cells deal with RNAs. In this presentation, I will discuss two recent studies exploring the regulatory mechanisms of exogenous RNAs.
In the first part, I will talk about our studies on viral RNA regulation. Using massively parallel reporter assays of viral genomic segments, we discovered hundreds of RNA elements that control RNA stability and translation. Investigation of their mechanisms provides new insights into the regulation of both viral and cellular RNAs. This research creates a valuable resource while highlighting the potential of viral genomes for biological discovery and therapeutic development.
Second, I will present our recent work on mRNA vaccines. Through genome-wide screens of in vitro-transcribed (IVT) mRNAs encapsulated in lipid nanoparticles (LNPs), we identified key cellular factors that impact mRNA. By comparing mRNAs with and without N1-methylpseudouridine modification, we also uncovered the mechanism by which this modification enhances protein production from IVT mRNAs. Our study provides a comprehensive map of cellular pathways regulating exogenous mRNAs, offering insights for improving RNA therapeutics and regulating RNA viruses.
Abstract
Inducible Hepatic-Associated Lymphoid Tissue (iHALT): liver as a surrogate secondary lymphoid organ in hepatotropic viral infection
John Gridley & Arash Grakoui
Emory University School of Medicine
Upon viral infection, the current paradigm of humoral immunity posits that germinal center (GC) reactions occurring within secondary lymphoid organs (SLOs) yield effector plasma cells that subsequently traffic via chemokine gradients to infected organs or bone marrow. However, it is not well understood how viral tissue tropism may govern the spatiotemporal dynamics of such responses during infection of various anatomical compartments. Contrasting a prototypical systemic virus with a strictly hepatotropic hepacivirus, we demonstrate that systemic infection induced liver-trafficking plasma cells generated in SLOs, whereas strictly hepatotropic infection elicited locally primed, virus-specific plasma cells within the liver independently of SLO contribution. Such locally derived progenies proceeded from inducible Hepatic-Associated Lymphoid Tissue (iHALT) structures harboring generative foci of Tfh, myeloid cells, and GC-like, blasting B cells bearing similar transcriptomic signatures to SLO GC dark zones. As functional iHALT was shown to be critical for viral resolution, this is the first known instance of humoral immunity exclusively generated and maintained within its extralymphoid site of viral infection amidst SLO dormancy, wherein iHALT is functionally successful in compensating for hepatotropic virus-induced SLO evasion strategies in preventing persistent infection.
Biography
Young Ki Choi
Managing Director / Director
Korea Virus Research Institute, IBS, Korea
choiki55@ibs.re.kr
Professor Choi obtained his B.S and M.S. from the College of Veterinary Sciences at Chungnam National University and went on to receive a Ph.D. in Virology at the University of Minnesota. During his post-doc fellow under the mentorship of Dr. Robert Webster at St. Jude Children’s Research Hospital, he experienced many laboratory and field studies on highly pathogenic zoonotic viral diseases. He has served as a professor at Chungbuk National University’s College of Medicine, where he conducted research on the pathogenic mechanisms by which viruses infect hosts as well as vaccine development. His recent studies on COVID-19 have garnered much attention at home and abroad, which include establishing the animal model of COVID-19 infection and transmission, as well as studying the antiviral treatment for COVID-19. From July 1, 2021, he has been conducting research on Emerging and re-emerging viral diseases as the managing director at the Korea Virus Research Institute (KVRI) in Institute of Basic Science (IBS). In KVRI, he is conducting research on the isolation and pathogenesis of emerging and re-emerging viral diseases and the development of novel platform therapeutics for infectious viral diseases.
Biography
SangJoon Lee
Assistant Professor
Ulsan National Institute of Science and Technology, Korea
sangjoon.lee@unist.ac.kr
Professor SangJoon Lee commenced his research career during his doctoral studies at the University of Tsukuba (Japan), investigating innate immunity against influenza infection. He subsequently undertook postdoctoral fellowships at the University of Tsukuba and St. Jude Children’s Research Hospital (USA), focusing on innate immunity against various viral infections and a form of cell death known as PANoptosis.
In 2022, Professor Lee assumed the role of Assistant Professor in the Department of Biological Science at the Ulsan National Institute of Science and Technology (UNIST), where his research has centered on inflammasomes and inflammatory cell death in the context of innate immunity, infectious diseases, inflammatory disorders, and cancer.
Throughout his career, Professor Lee has been the recipient of numerous prestigious awards and honors, including the HBP Fellow Award in 2013 (University of Tsukuba), the Outstanding Research Award (University of Tsukuba) in 2019, the Yuhan Innovation Program Award (Yuhan Corporation) in 2022, and appointment as Director of the UNIST Pandemic Research Center in 2023 (The Circle Foundation).
Biography
Yong Taik Lim
Professor
Sungkyunkwan University, Korea
yongtaik@skku.edu
Prof. LIM is currently a Professor at the Department of Nano Engineering and SKKU Advanced Institute of Nanotechnology in Sungkyunkwan University (SKKU), Suwon, Korea. His research field of interest is Immuno-Bio-Engineering for Cancer Immunotherapy and Infectious Disease. He is developing designer biopharmaceuticals for cancer immunotherapy and vaccine adjuvants for infectious disease.
After he received his Ph.D. degree at the Department of Chemical and Biomolecular Engineering in the Korea Advanced Institute of Science and Technology (KAIST) in 2002, he joined John V. Frangioni’s laboratory at Harvard Medical School as a postdoctoral research fellow. He also worked about 5 years at the two Korea government-supported research institutes (ETRI and KRIBB) as an alternative military service. He started professorship at Chungnam National University in 2009 and moved to Sungkyunkwan University in 2014.
He received the B.S. degree from the department of the Chemical Engineering at the Sogang University, Korea in 1996. He then received the M.S. degree from the department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) in 1998 and Ph.D. at KAIST in 2002.
Biography
Nam-Hyuk Cho
Professor
Seoul National University, Korea
chonh@snu.ac.kr
Dr. Nam-Hyuk Cho has a longstanding interest in host-pathogen interactions, particularly in emerging human pathogens. He is currently a professor in the Department of Microbiology and Immunology at Seoul National University College of Medicine, Seoul, South Korea. Dr. Cho began studying immune responses and immunopathogenesis during the infection of Orientia tsutsugamushi, the causative agent of scrub typhus, during his Ph.D. training at Seoul National University. He expanded his research areas to virology while undergoing postdoctoral training at Harvard Medical School. Dr. Cho has a broad background in microbiology, virology, cellular immunology, and molecular biology. He has collaborated with clinicians in several Korean hospitals to study the immunological pathogenesis of several endemic and new emerging infectious diseases, including scrub typhus, severe fever with thrombocytopenia syndrome (SFTS), and emerging coronavirus infections.
Dr. Cho’s team investigates potential vaccine antigens through both animal model studies and clinical research on scrub typhus. Their research also delves into virus-host cell interactions, with a focus on emerging viral pathogens such as SFTSV, MERS-CoV, and SARS-CoV-2. By elucidating the fundamental mechanisms these pathogens employ, their work not only advances strategies for combating severe infections but also deepens our understanding of immune system evolution. Ultimately, Dr. Cho seeks to engineer immune structures through a novel approach termed immunoarchitectonics, aiming to regulate immune responses against a range of human diseases, including infections and cancers, by modulating the functional architecture of the immune system.
Biography
Eui-Cheol Shin
Professor
Korea Advanced Institute of Science and Technology, Korea
ecshin@kaist.ac.kr
Dr. Eui-Cheol Shin is a Professor at Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Republic of Korea. His lab investigates T cell responses in human viral disease and cancer with a focus on CD8+ T cells. In particular, his lab studies cytokine-mediated, TCR-independent activation of CD8+ T cells that contribute to host cell injury. A series of his studies has revived the research of bystander T cell activation in microbial infections as a cause of immunopathologic host injury (Nat Immunol 2022, 23:13).
During the COVID-19 pandemic, his lab has also contributed to the understanding of human immune responses to SARS-CoV-2 infection and vaccination. Particularly, his lab revealed the successful generation of stem cell-like memory T cells after SARS-CoV-2 natural infection or vaccination, the preserved functionality of vaccine-induced memory T cells against SARS-CoV-2 variants, and the evolution of human T cell responses following breakthrough infections with new variants. Through these studies, his lab proposed immunological strategies for the control of the pandemic (Nat Rev Immunol 2020, 20:585; and Nat Rev Immunol 2021, 21:687).
He received M.D. (1996) and Ph.D. (2001) from Yonsei University College of Medicine, Seoul, Republic of Korea, and was trained as a postdoctoral fellow at NIDDK, National Institutes of Health, Bethesda, Maryland, USA (2002 – 2007). Then he joined Graduate School of Medical Science and Engineering, KAIST in 2007, where he is currently a Professor. During the COVID-19 pandemic, he served as the director of the Center for Viral Immunology, Korea Virus Research Institute, Institute for Basic Science (IBS), Daejeon, Republic of Korea (2021 – 2024). He was elected the Fellow of the Korea Academy of Science and Technology (2019) and the Fellow of the National Academy of Medicine of Korea (2024).
Biography
Atsushi Kawaguchi
Professor
University of Tsukuba, Japan
ats-kawaguchi@md.tsukuba.ac.jp
Dr. Atsushi Kawaguchi is a Professor in the Department of Infection Biology, Institute of Medicine, University of Tsukuba, Japan. He also serves as Director of the Transborder Medical Research Center at University of Tsukuba, where he leads multidisciplinary research initiatives in infectious disease. He received his PhD in 2007 from University of Tsukuba. After five years of postdoctoral training at University of Tsukuba and Kitasato University, Dr. Kawaguchi joined the faculty of University of Tsukuba in 2012 as an Assistant Professor. He was promoted to Associate Professor in 2018 and to Professor in 2020. His research has been focused on the molecular and cellular mechanisms of virus-host interactions, particularly in influenza viruses and SARS-CoV-2 infection. His group investigates viral RNA synthesis, intracellular trafficking, innate immune response, and pathogenesis based on molecular biology techniques, genetically modified mouse models, and advanced imaging technologies.
Biography
Min-Suk Song
Professor
Chungbuk National University, Korea
songminsuk@chungbuk.ac.kr
Min-Suk Song, Ph.D. is a Professor in the College of Medicine and the Medical Research Institute at Chungbuk National University (Cheongju, Korea). He is a microbiologist specializing in influenza pathogenesis and the development of antivirals and vaccines.
Dr. Song earned a B.S. in Biology (2006) and an M.S. in Microbiology (2008) from Chungbuk National University, and completed his Ph.D. there in 2011 on the pathogenic determinants of influenza viruses. He was a postdoctoral research associate at Chungbuk National University (2011–2012) and at St. Jude Children’s Research Hospital, USA (2013–2014). He joined Chungbuk National University as an Assistant Professor in 2014, became Associate Professor in 2018, and Professor in 2023.
His current work examines antiviral efficacy, resistance, and drug combinations in vitro and in vivo, and leads a capless self-amplifying RNA vaccine program. He is a member of the Korean Society for Microbiology and Biotechnology, the Korean Society of Virology, the American Society for Virology, and the Microbiology Society.
Min-Suk Song, Ph.D. is a Professor in the College of Medicine and the Medical Research Institute at Chungbuk National University (Cheongju, Korea). He is a microbiologist specializing in influenza pathogenesis and the development of antivirals and vaccines.
Dr. Song earned a B.S. in Biology (2006) and an M.S. in Microbiology (2008) from Chungbuk National University, and completed his Ph.D. there in 2011 on the pathogenic determinants of influenza viruses. He was a postdoctoral research associate at Chungbuk National University (2011–2012) and at St. Jude Children’s Research Hospital, USA (2013–2014). He joined Chungbuk National University as an Assistant Professor in 2014, became Associate Professor in 2018, and Professor in 2023.
His current work examines antiviral efficacy, resistance, and drug combinations in vitro and in vivo, and leads a capless self-amplifying RNA vaccine program. He is a member of the Korean Society for Microbiology and Biotechnology, the Korean Society of Virology, the American Society for Virology, and the Microbiology Society.
Biography
Rajendra Karki
Assistant Professor
Seoul National University, Korea
rkarki@snu.ac.kr
Prof. KARKI is currently an Assistant Professor at Seoul National University in Seoul, Korea. His research field of interest is innate immunity and inflammatory cell death.
From 2013-2023, he worked in the Department of Immunology at St. Jude children’s Research Hospital in Tennessee, USA. During his time there, he progressed from Postdoctoral Fellow to Staff Scientist and eventually served as a Lab Director. In 2023, he joined Seoul National University as an Assistant Professor. He serves on the editorial boards of Frontiers in Immunology, Frontiers in Pharmacology, and PLoS Pathogens.
His research has defined the molecular logic of inflammasome activation and cell death plasticity, establishing mechanistic frameworks that inform both fundamental biology and translational immunology. He was named a Highly Cited Researcher by Clarivate (2024), placing him among the top 1% of scientists globally in the field of Immunology. According to Scholar GPS (2023 and 2024), he ranks among the top 10 most influential researchers in inflammasome biology. He is also the recipient of the AAI–Thermo Fisher Trainee Achievement Award and the Milstein Travel Award from the International Cytokine and Interferon Society (ICIS).
He received the B.S. degree in Pharmaceutical Sciences at the Pokhara University, Nepal in 2005. He then received the M.S. degree from the Department of Oriental Medicine at Mokpo National University in 2008 and Ph.D. degree from the same University in 2011.
Biography
Benjamin Brennan
Senior Lecturer & Group Leader
University of Glasgow, UK
ben.brennan@glasgow.ac.uk
Dr. BRENNAN is currently a Senior Lecturer and Group Leader at the MRC-University of Glasgow Centre for Virus Research, United Kingdom. His research field of interest is in molecular arbovirology, with particular focus on bunyaviruses.
He started his professional career as a postdoctoral research associate at the University of St. Andrews in the laboratory of the late Prof Richard Elliott (2008-2013) and later moved to the University of Glasgow (2013-2018). In 2018, he received a prestigious Wellcome Trust Sir Henry Dale fellowship, which supported his transition to an independent research career. He is currently a Senior Lecturer at the University of Glasgow’s Centre for Virus Research (CVR), where he leads a research programme focused on the molecular biology and host interactions of arthropod-borne viruses, particularly phleboviruses and bunyaviruses. His work integrates reverse genetics, vector biology, and immunology to elucidate the mechanisms of virus replication, host tropism, and transmission dynamics in both mammalian and arthropod systems. His scholarly output includes over 30 peer-reviewed publications, with more than 1,700 citations and an h-index of 22.
In addition to his research, Dr. Brennan is actively involved in public engagement initiatives, including a citizen science project aimed at mapping tick populations and associated viruses in Scotland. He also serves on the Virus Division of the Microbiology Society and as an Editor for the Journal of General Virology, contributing to the broader virology community.
He received his BSc degree in Medical Microbiology in 2005 and later his PhD in 2008 from the University of Surrey, UK.
Biography
Jong-Eun Park
Associate Professor
Korea Advanced Institute of Science and Technology, Korea
jp24@kaist.ac.kr
My research is focused on understanding how the information stored in genome can be interpreted and expressed to coordinate diverse biological phenomenon. During my PhD training, I studied non-coding RNAs and the mechanism post-transcriptional regulations, including miRNA biogenesis, poly(A) tail mediated translational regulation during cell cycle and ncRNA processing by nuclear deadenylases. As I joined the Dr. Sarah Teichmann’s lab in Sanger Institute, I actively participated in the Human Cell Atlas from its beginning. I developed batch correction method which can applied onto large scale single-cell dataset. I also led the Human thymus atlas project, reconstructing the development and aging of human organ at single-cell resolution. Since 2020, I became independent researcher at the Graduate School of Medical Science and Engineering, KAIST, where I’m applying single-cell omics to understand complex phenomenon such as aging, cancer, and autoimmune disorders, especially focusing on the large-scale data integration and interpretation.
Biography
Jin-Hyun Ahn
Professor
Sungkyunkwan University, Korea
jahn@skku.edu
Jin-Hyun Ahn received his B.S. and M.S. in Microbiology at the Department of Microbiology of Seoul National University in Seoul, South Korea. He earned his Ph.D. in Microbial Genetics in the same Department. He completed his postdoc training at Johns Hopkins University School of Medicine in the laboratory of Gary S. Hayward. After that, he joined the faculty at the Sungkyunkwan University School of Medicine in Suwon, South Korea, where he is now a Professor in the Department of Microbiology and serves as Director of the Institute of Basic Medicine. His research examines the molecular interactions between herpesviruses and their hosts, with a focus on how viral gene products influence host intrinsic defense and innate immune responses. He also studies the viral regulation of cellular ubiquitin and ubiquitin-like modification systems, as well as the functions of G-quadruplexes formed in the genomes of herpesviruses. He served as President of the Korean Society of Virology.
Biography
Moon Jung Song
Professor
Korea University, Korea
moonsong@korea.ac.kr
Prof. Moon Jung Song is currently a Professor in the Department of Biotechnology and Director of the Korea BioDefense Research Institute (KBDRI) at Korea University, Seoul, Republic of Korea. Her research focuses on molecular mechanisms underlying virus-host interactions, viral immune evasion strategies, and the identification of novel targets for antiviral therapeutics, trying to bridge fundamental virology with translational applications in infectious disease control.
She began her professional career as an Assistant Professor at Hallym University College of Medicine (2004-2006) before joining Korea University in 2006, where she has held the leadership positions as the Vice President of Graduate Office of Korea University (2024-2025), and Associate Dean for Academic & Student Affairs (2020-2022), and Deputy Director and Director of KBDRI (2017-2020; 2021~).
Prof. Song’s laboratory has made significant contributions to understanding the molecular interplay between viruses and host cellular machinery, with recent work on viral G-quadruplex formation and how viruses manipulate cellular processes such as immune recognition, poly(ADP-ribose) polymerization, and NAD+ metabolism published in Nucleic Acids Research, Journal of Cell Biology, PLoS Pathogens, Journal of Virology, and Journal of Medical Virology. She maintains active research collaborations with leading institutions worldwide. She is currently leading a Korea-US collaborative research project with The Broad Institute of MIT and Harvard.
She actively contributes to the scientific community as Academic Chair for Korean Society of Virology, Treasurer of the Korean Society for Molecular and Cellular Biology, Chairs in Women Bioscience Forum, Chair of Education Committee for the Korea Federation of Women’s Science and Technology Associations, and Associate Editor for Frontiers in Microbiology. Prof. Song has received prestigious awards including the Prime Minister’s Commendation for Science and Technology Promotion (2023) and the MSK Women Scientists Award (2019). She has also been recognized for her teaching excellence with multiple ‘Seok Tap Teaching Excellence Awards’ and ‘Distinguished Teaching Awards’ from Korea University.
She received her B.S. and M.S. degrees from College of Pharmacy, Seoul National University and earned her Ph.D. in Molecular and Medical Pharmacology from School of Medicine UCLA in 2002, where she also completed her postdoctoral research (2002-2004).
Biography
Heeju Ryu
Assistant Professor
Sungkyunkwan University, Korea
heejuryu@skku.edu
Dr. Ryu is an Assistant at Sungkyunkwan University in Suwon, South Korea. Her research aims to understand the link between immune cell function and disease to develop new therapeutic agents and predictive response models.
She earned her B.S. in Chemistry with Distinction from the University of Michigan in 2013 and her Ph.D. from the College of Pharmacy at Seoul National University in 2019. After completing her postdoctoral training at the Fred Hutchinson Cancer Center, she began her appointment at Sungkyunkwan University School of Medicine in 2024.
Biography
Seung-Woo Lee
Professor
Pohang University of Science and Technology, Korea
sw_lee@postech.ac.kr
Prof. LEE is currently a Professor at POSTECH (Pohang University of Science and Technology), Pohang, Korea. His research field of interest is mucosal immunology and immunotherapy.
He started his professional career as an Assistant Professor in Department of Life Sciences at the POSTECH where he was later appointed as a Professor. He became the director of Microbiome Research Center at POSTECH in 2020, and has served several positions at the Korea Association of Immunologist.
He has received various awards such as Achievement Award (Ministry of Health and Welfare) in 2024, KAI-Genexine Grand Achievement Award in 2022, and Achievement Award (Ministry of Science and ICT) in 2021.
He received the B.S. degree from the department of Life Sciences at POSTECH, Korea in 1994. He then received the Ph.D. degree from the same department at POSTECH 2000.
Biography
Min Kyung Jung
Research fellow
Korea Virus Research Institute, IBS, Korea
mkjung@ibs.re.kr
Dr. Min Kyung Jung is a research fellow at the center for viral immunology, Korea Virus Research Institute (KVRI), Institute for Basic Science (IBS), Daejeon, Korea. Her research focuses on human T cell immunity elicited by viral infections and vaccination, with particular emphasis on the phenotypes and functions of memory T cells and their roles in disease pathogenesis and immune protection.
She began her career as a research professor at the department of life science, Sookmyung Women’s University (2010–2014). She then served as a research assistant professor at the graduate school of medical science and engineering, KAIST (2014–2021), where she continued investigating T cell responses in viral hepatitis and other infectious diseases. From 2018 to 2020, she was also a research fellow in the Immunology Section, Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH), Bethesda, USA, studying liver-resident and bystander T cells in chronic viral hepatitis. Since December 2021, she has led research on T cell responses following SARS-CoV-2 infection and vaccination, contributing to the understanding of immune memory and cross-reactivity to variants of concern. She has shown that BNT162b2-induced memory T cells retain robust polyfunctionality against the Omicron variant (Nat Microbiol), and that Omicron breakthrough infections elicit CD8+ T cell responses against later Omicron subvariants (Sci Immunol).
Biography
Yeonseok Chung
Professor
Seoul National University, Korea
yeonseok@snu.ac.kr
Prof. Chung is currently a Professor of Immunology at Seoul National University, Seoul, Korea. His research field of interest includes immunometabolism, immunosenescence and tissue immunity.
He started his professional career as an Assistant Professor in Institute of Molecular Medicine, The University of Texas medical school at Houston (2010-2014), and moved to Seoul National University in 2014. In 2024, he was appointed as the chair innovative pharmaceutical sciences at the newly-launched College of transdisciplinary innovations at Seoul National University. He has served as chair/vice-chair of academic affair at various academic societies such as the Korean Association of Immunologists (KAI), Pharmaceutical Society of Korea (PSK), Federation of Immunological Societies of Asia-Oceania (FIMSA).
He has received various prestige awards such as KAI-Genexine Excellence Award in 2018 (KAI), 2010 Seymour and Vivian Milstein Young Investigator Award (2010), Herbert Tabor Young Investigator Award (2014), Top 100 National Research and Development Achievements (2019, Ministry of Science and ICT of Korea), and Leader Researcher Award (2020, NRF Korea).
He received the B.S. degree from the department of Pharmacy at the Seoul National University, Korea in 1997. He then received the Ph.D. degree from the same University in 2023.
Biography
Masanori Isogawa
Director
National Institute of Infectious Diseases, Japan
nisogawa@niid.go.jp
Dr. Masanori Isogawa, M.D., Ph.D., graduated from Mie University School of Medicine in 1998. Following a brief residency in Internal Medicine, he started his research career at the Institute for Virology in Essen University Hospital in 1999. In 2001, he relocated to La Jolla, CA, USA, to join the Chisari Laboratory at the Scripps Research Institute (TSRI), renowned for its groundbreaking work in hepatitis research. Utilizing various mouse models of hepatitis B virus (HBV) infection, Dr. Isogawa made seminal contributions to elucidating the impact of intrahepatic antigen recognition on HBV-specific CD8 T cells. These achievements led to his appointment as Assistant Professor at TSRI. With Dr. Chisari, Dr. Isogawa then generated HBV-specific T cell receptor transgenic mice, uncovering that HBV-specific T cell priming in the liver induces T cell tolerance. This model, established by him and adopted by other research groups, significantly advanced our understanding of liver-mediated T cell tolerance. In 2013, Dr. Isogawa returned to Japan to join Dr. Tanaka’s group at Nagoya City University, one of Japan’s leading laboratories in HBV research. In 2020, he moved to the Department of Immunology at the National Institute of Infectious Diseases, focusing on dissecting immune responses to COVID-19 infections and vaccinations. In 2024, Dr. Isogawa was appointed to his current position, where he directs basic research on hepatitis and diarrhea viruses. His primary interests lie in unraveling the molecular and cellular mechanisms of chronic virus infections and in the development of therapeutic vaccines against chronic HBV infection.
Biography
You-Me Kim
Associate Professor
Korea Advanced Institute of Science and Technology, Korea
youmekim@kaist.ac.kr
Dr. You-Me Kim is an Associate Professor at the Graduate School of Medical Science and Engineering at KAIST in Korea. She received her B.S. in Pharmacy and her M.S. in Pharmacology from Seoul National University, and went on to earn her Ph.D. in Molecular Pharmacology and Structural Biology from Thomas Jefferson University in the United States. Following her doctoral training, she pursued postdoctoral research at Harvard Medical School and the Whitehead Institute for Biomedical Research, where she investigated innate and adaptive signaling pathways regulating immune functions. Dr. Kim then joined the Novartis Institutes for Biomedical Research in Cambridge, Massachusetts, where she contributed to early-stage drug discovery efforts focused on immunology and inflammation. After returning to Korea in 2009, she joined the Department of Life Sciences at POSTECH, where she continued her research on immune regulation and mucosal immunology. In 2018, she was appointed to her current position at KAIST.
Her research program is centered on elucidating the cellular and molecular mechanisms that regulate immune cell homeostasis, activation, and differentiation under both physiological and pathological conditions. A major focus of her current work is on the crosstalk between the host immune system and commensal microbiota, and how dysregulation of this interaction contributes to chronic inflammatory and autoimmune diseases. Her lab employs a wide range of experimental approaches including in vivo disease models, cellular immunology, and molecular genetics to uncover novel therapeutic targets and pathways. Through her research, Dr. Kim aims to build a comprehensive framework for understanding immune regulation in complex tissue environments and to contribute to the development of innovative strategies for the treatment of immune-mediated disorders.
Biography
Bertram Bengsch
Professor
University of Freiburg, Germany
Bertram.bengsch@uniklinik-freiburg.de
Prof. Bengsch is currently the Section Head for Translational Systems Immunology in Hepatogastroenterology at the University Medical Center (UMC) Freiburg, Germany. His research centers on understanding the regulation of T cell immunology in infection, autoimmunity, cancer and during immunotherapy
Following his studies of medicine in Freiburg, London and Philadelphia, he started his professional career as Clinician Scientist at the UMC Freiburg (2009-2014). As a postdoctoral Research Fellow he moved to the University of Pennsylvania (Institute of Immunology & Parker Institute for Cancer Immunotherapy). He was appointed Heisenberg Professor for Translational Hepatogastroenterology at the UMC Freiburg in 2020, where he is also heading the Liver cancer outpatient clinic and the Freiburg Mass Cytometry Facility. He is also serving as chair and board member in several academic societies such as the T cell subsets group of the German Society for Immunology, the German Mass Cytometry Network and the German Society for Cytometry.
His work has been seminal to identify the role of metabolic regulation for T cell exhaustion and to elucidate mechanisms of successful immunotherapies. He has received several individual research prizes and has been repeatedly ranked as Highly Cited Researcher.
He received the M.D. degree from the University of Freiburg in 2008 and received his Dr. med. from the Faculty of Medicine studying the CD8+ T cell differentiation in Hepatitis B and C virus infection. He received the Ph.D. degree from the Faculty of Biology, University of Freiburg in 2013. He is a board certified Internist and Gastroenterologist.
Abstract
Animal organoid model systems and their applications for studying of emerging and re-emerging viruses
Hyunjoon Kim1, Seo-Young Heo1, Young-Il Kim1 Bon-Kyoung Koo2, Young Ki Choi1
1Center for Study of Emerging and Re-emerging Viruses, Korea Virus Research Institute, 2Center for Genome Engineering, Institute for Basic Science, Institute for Basic Science, Daejeon 34126, Republic of Korea
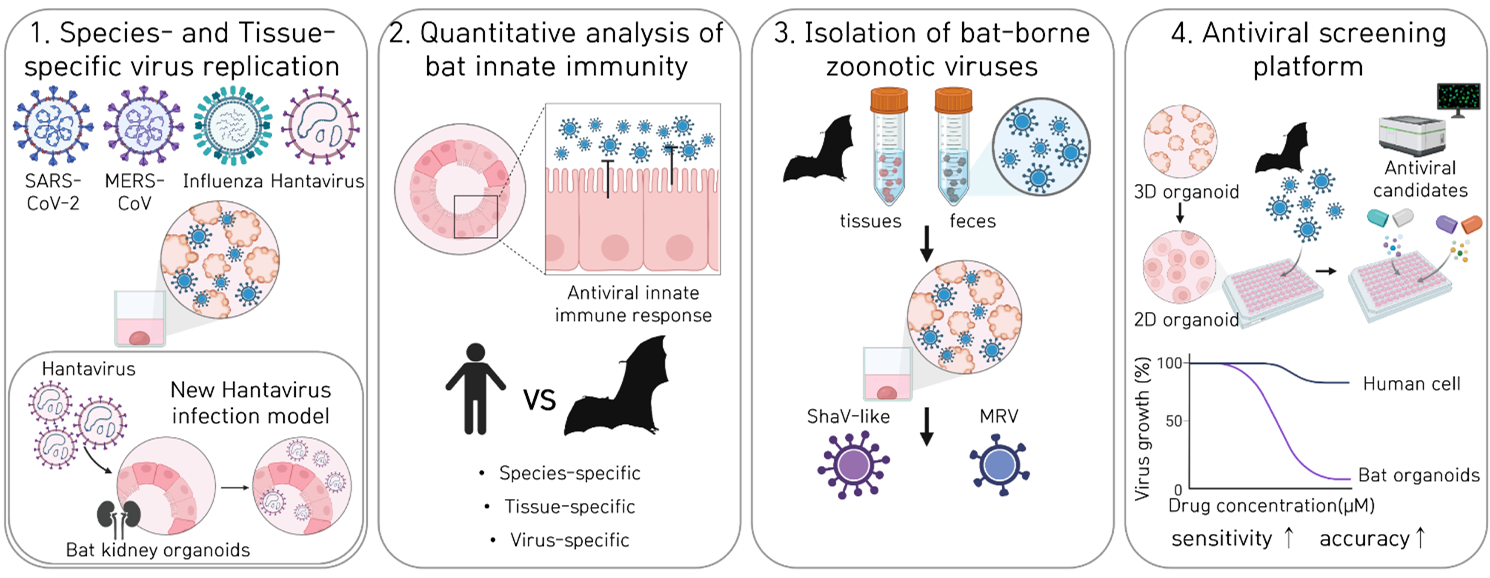
Zoonosis, an infectious disease that transmits from animals to humans, accounts for many historical outbreaks including COVID-19. Bats are well-recognized as key reservoirs for zoonotic pathogens, yet appropriate model systems to study these interactions have been limited. This gap hinders our ability to explore host-pathogen interactions and assess spillover risks comprehensively. To address this, we developed a collection of bat organoid models that encompass five species and three distinct organ types. With this model system, we could isolate and characterize bat-borne mammalian orthoreovirus and novel paramyxovirus, demonstrating their utility for simultaneous virome monitoring. These multi-species and multi-organ bat organoids present species- and tissue-specific replication patterns of multiple pathogenic virus families, including avian influenza viruses, revealing the zoonotic potential of avian-to-bat transmission. Overall, our innovative organoid platform offers a robust method for screening, isolating, and characterizing novel viruses, enhancing our ability to preempt and mitigate pandemic risks posed by bat-borne diseases.
Figure 1. Species- and tissue- specific replication, innated immunity, anti-viral responses of of zoonotic viruses, in multi-species and multi-organ bat organoids.
- Kim H, S Heo, Y Kim et al, Diverse bat organoids provide pathophysiological models for zoonotic viruses. Science. 2025 May 15;388(6748):756-762.
- Kim H, Koo BK, Choi YK. Mini bat organs reveal hidden viral threats. Clin Transl Med. 2025 Aug;15(8):e70454
Abstract
AIM2 Inflammasome as a Double-Edged Sword in Viral Immunity
SangJoon Lee
Department of Biological Science, UNIST, Ulsan 44919, Republic of Korea
The absent in melanoma 2 (AIM2) inflammasome functions as a central innate immune sensor for cytosolic DNA and plays a crucial role in antiviral defense. However, its activation can lead to divergent outcomes, ranging from effective viral clearance to excessive inflammation and host tissue damage. In this study, we investigate the dual role of AIM2 in two distinct viral contexts: monkeypox virus (MPXV) and herpes simplex virus type 1 (HSV-1). Using a systematic knockout screen and infection models, we identify AIM2 as the dominant inflammasome sensor responsible for detecting MPXV. Upon activation, AIM2 assembles a multi-protein complex with ASC and caspase-1, leading to pyroptosis in infected cells and triggering apoptosis and necroptosis in bystander cells. These AIM2-mediated cell death pathways collectively restrict viral spread but also contribute to lung inflammation and immunopathology in vivo. In a highly susceptible mouse model, pharmacological inhibition of AIM2 partially rescued survival, highlighting its therapeutic relevance. In parallel, we demonstrate that HSV-1 strain-specific differences in AIM2 activation are determined by the presence of unique poly(T) DNA sequences in the HF strain genome. These sequences act as potent ligands for AIM2 and are sufficient to drive inflammasome activation, cytokine release, and pyroptotic cell death. Strains lacking these sequences (such as F and KOS) fail to activate AIM2, and deletion of the poly(T) motifs in the HF strain abrogates host protection. Together, our findings establish AIM2 as a double-edged sword in viral immunity—essential for sensing viral DNA and initiating host defense, but also capable of mediating inflammatory damage depending on viral context and ligand availability. These insights provide a foundation for targeting AIM2 in the development of antiviral and anti-inflammatory therapies.
- Herpes Simplex Virus 1 Harboring Poly(T) DNA Sequences as a Key Ligand for AIM2 Inflammasome Activation and Host Defense. S. Oh, J. Oh, J. Lee, K. Im, T. Kim, K. Shin, N. Mariam, C. Seok, J. Park, G. Yu, H. Kim, S. Kim, S. Shin, D. Kim, Y. Choi*, E. Kim*, J. Lee*, and S. Lee* (*Correspondence). Nature Communication (IF: 14.7), Under Revision.
- AIM2 drives inflammatory cell death and monkeypox pathogenesis. J. Oh, Y. Hwang, J. Lee, C. Seok, S. Oh, H. Kim, N. Mariam, J. Ahn, G. Yu, J. Park, H. Kim, S. Kim, S. Shin, J. Lee, Y. Choi, D. Kim, D. Kim, Y*. Kim*, and S. Lee* (*Correspondence). Cellular & Molecular Immunology (IF: 21.8), Under Revision.
- Inflammasome diversity: Exploring novel frontiers in the innate immune response. G. Yu, Y. Choi, and S. Lee* (*Correspondence). Trends in Immunology (IF: 13.1), March. 21. 2024.
- Integrated NLRP3, AIM2, NLRC4, Pyrin inflammasome activation and assembly drive PANoptosis. S. Oh, J. Lee, J. Oh, G. Yu, H. Ryu, D. Kim, and S. Lee* (*Correspondence). Cellular & Molecular Immunology (IF: 21.8) Nov. 27, 2023.
- AIM2 forms a complex with Pyrin and ZBP1 to drive PANoptosis and host defense. S. Lee, R. Karki, Y. Wang, L. Nguyen, R. Kalathur, and T.D. Kanneganti. Nature (IF: 69.5), Sept. 01, 2021.
Abstract
Immune Designer Nano-vaccines for Reshaping Dynamic Immunity
Yong Taik Lim
SKKU Advanced Institute of Nanotechnology (SAINT), Department of Nano Science and Technology, Department of Nano Engineering, School of Chemical Engineering, and Biomedical Institute for Convergence at SKKU, Sungkyunkwan University, Suwon, Republic of Korea. E-mail address: yongtaik@skku.edu
Immunity is determined by the spatiotemporal distribution of antigens/adjuvants and the need for safe vaccines that ensure long-term and broad protection against multiple viral variants has been increased after the emergence of catastrophic infectious diseases. As a next-generation vaccine against emerging infectious risks, here, we suggest designer immunomodulatory nano-vaccines that mimic natural infection in allowing fine-tuned dynamic control over the dosage, timing, and location of antigens/adjuvants at both the cellular and tissue levels [1-4]. Herein, we suggest two-types of novel engineered nanoadjuvants (SE(Trojan-TLR7/8a) and alum-Trojan-TLR7/8a) that evoke germinal centre (GC) B cells and polyfunctional T cells via multiscale kinetic immunomodulation through clinically approved platform (squalene nanoemulsion or alum)-mediated macroscopic control of vaccine delivery and the Trojan-TLR7/8a-enabled timely activation of antigen-presenting cells (APCs) [1-2]. Engineered nanoadjuvants enhance the migration of non-exhausted APCs into lymph nodes and elicit activation of follicular helper T cells and the generation of GC B cells and polyfunctional T cells. Engineered nanoadjuvants outperform current commercial vaccine adjuvants (Alum, AS03, or mRNA vaccines) and demonstrate cross-protection against diverse influenza and SARS-CoV-2 variants, ensuring 100% protection with a healthy state. Engineered nanoadjuvants also sustain a potent T cell response in an aged ferret model of SFTSV infection, demonstrating long-term and broad protective immunity against emerging pandemic and epidemic infectious viruses.
- Yeon Jeong Yoo et al, and Yong Taik Lim*, “Multiscale dynamic immunomodulation by a nanoemulsified Trojan-TLR7/8 adjuvant for robust protection against heterologous pandemic and endemic viruses”, Cellular & Molecular Immunology, 2025, in press.
- Sang Nam Lee et al, and Yong Taik Lim*, “Kinetically activating nanovaccine mimicking multidimensional immunomodulation of natural infection for broad protection against heterologous viruses in animal models”, Nature Communications, 2025, 16, Article number: 2914
- Seung Mo Jin et al, and Yong Taik Lim*, “Spatiotemporal Dynamic Immunomodulation by Infection-Mimicking Gels Enhances Broad and Durable Protective Immunity Against Heterologous Viruses”, Advanced Science, 2025, 12, 2412116
- Seung Mo Jin et al, and Yong Taik Lim*, “Transformable Gel-to-Nanovaccine Enhances Cancer Immunotherapy via Metronomic-like Immunomodulation and Collagen-mediated Paracortex Delivery”, Advanced Materials, 2024, 36, 2409914
Abstract
Recombinant Bandavirus as an effective bivalent vaccine platform inducing protective immunity against intracellular pathogen and cancer
Nam-Hyuk Cho
Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul 03080, Republic of Korea
Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne pathogen endemic to East Asia, associated with high mortality and lacking approved vaccines or therapies. We developed a live attenuated vaccine platform based on a recombinant SFTSV ΔNS virus, which lacks the nonstructural (NS) gene-a key virulence factor that suppresses type I interferon responses. The resulting ΔNS viruses, reassorted with the prevalent genotype B, showed attenuated replication in interferon-competent cells while retaining the ability to infect and activate antigen-presenting cells (APCs). Immunization with the ΔNS virus elicited robust SFTSV-specific humoral and cellular immune responses in mice and non-human primates, conferring complete protection against lethal challenge across multiple SFTSV genotypes, with immunity lasting up to 12 months. Additionally, recombinant ΔNS viruses encoding heterologous antigens, such as ovalbumin (OVA) or TSA56 from Orientia tsutsugamushi, induced strong antigen-specific T cell responses and conferred protection against OVA-expressing melanoma or scrub typhus, respectively. Mechanistically, ΔNS viruses enhanced APC activation, improved antigen presentation, and reduced apoptosis in infected cells, supporting effective T cell priming. These findings establish SFTSV ΔNS as a safe, immunogenic, and broadly protective vaccine candidate, and a versatile bivalent vector platform for targeting viral, intracellular bacterial, and tumor-associated antigens.
Abstract
Regulation of IL-15-induced NK-like activation of CD8+ T cells in viral infection
Eui-Cheol Shin
Graduate School of Medical Science and Engineering, KAIST, Daejeon 34051, Republic of Korea
During viral infection, pre-existing memory CD8+ T cells that are not specific for the infecting virus can be activated by cytokines without cognate antigens, termed bystander activation (Kim et al. Immunity 2018, 48:161-173). Bystander-activated CD8+ T cells exert either protective or detrimental effects on the host depending on the infection model or disease (Lee et al. Nat Immunol 2022, 23:13-22). Recently, my laboratory investigated regulatory mechanisms of TCR-independent bystander CD8+ T cell activation. We found that TCR signals suppress characteristic features of IL-15-induced CD8+ T cell activation, including the increased NKG2D expression and upregulation of genes related to NK cell-mediated cytotoxicity and IFN response. Furthermore, we found that Ca2+/calcineurin signaling pathway is responsible for TCR-mediated suppression of IL-15-induced bystander activation. Interestingly, calcineurin inhibitors could not suppress IL-15-induced bystander activation and paradoxically increased IL-15-induced NKG2D expression in the presence of TCR signals. Additionally, we defined genes upregulated by IL-15 and downregulated by concurrent TCR signals as a “bystander activation-specific gene set” and found the upregulation of this gene signature in bystander CD8+ T cells from patients with hepatitis A virus infection, a prototype disease with immunopathology mediated by bystander-activated CD8+ T cells. Our study paves the way for further investigation of bystander CD8+ T cell activation in various pathological conditions and its regulation.
Abstract
Molecular basis of SARS-CoV-2 multiorgan infection via viremia
Yukino Ogura, Atsushi Kawaguchi
Department of Infection Biology, Institute of Medicine, University of Tsukuba, Tsukuba 305-8575, Japan
Coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), primarily induces acute respiratory symptoms. However, in severe cases, multiple organ failure frequently occurs—including cardiovascular complications and acute kidney injury—as the SARS-CoV-2 virus can enter the bloodstream and directly infect various organs. COVID-19-associated cardiac complications have been reported in approximately 12 to 35% of hospitalized patients, ranging from mild palpitations and chest pain to severe conditions including myocarditis and heart failure. These complications have also been linked to long-term cardiac dysfunction, potentially involving myocardial fibrosis, even after recovery from COVID-19.
The bloodstream is separated from the alveolar sacs to ensure efficient gas exchange via the blood-air barrier, which is composed of alveolar epithelial cells, basement membrane, and capillary endothelial cells. The basement membrane is a specialized extracellular matrix (ECM) that includes collagen IV, laminins, and proteoglycans. This network of collagen fibers and proteoglycans forms a dense gel-like structure that serves as a physical barrier against external particulate substances and infectious agents. SARS-CoV-2 has been reported to directly infect alveolar epithelial and endothelial cells, leading to the loss of epithelial integrity. However, the precise mechanism by which SARS-CoV-2 disrupts the basement membrane remains unclear.
Here, we demonstrate that SARS-CoV-2 infection induces invadopodia formation—actin-rich protrusions specialized for extracellular matrix degradation—and promotes the matrix metalloproteinase (MMP)-mediated invasive ability of infected cells. Viral spike protein mediates invadopodia formation, which act as local sites for viral replication. This virus-induced invadopodia formation is regulated by CD147, a membrane protein also involved in cancer cell invasion. Moreover, administration of an MMP inhibitor suppressed direct SARS-CoV-2 infection in the heart in vivo.
Abstract
A SARS-CoV-2 Mpro mutation conferring ensitrelvir resistance paradoxically increases nirmatrelvir susceptibility
Min-Suk Song
Department of Microbiology, Chungbuk National University College of Medicine and Medical Research Institute, Cheongju, Chungbuk, 28644, Republic of Korea
SARS-CoV-2 variants resistant to current antivirals remain a significant threat, particularly in high-risk patients. Although nirmatrelvir and ensitrelvir both target the viral 3CL protease (Mpro), their distinct susceptibility profiles may allow alternative therapeutic approaches. Here, we identified a deletion mutation at glycine 23 (Δ23G) in Mpro that conferred high-level resistance to ensitrelvir (~35-fold) while paradoxically increasing susceptibility to nirmatrelvir (~8-fold). This opposite susceptibility pattern was confirmed both in vitro and in a hamster infection model. Recombinant viruses carrying Mpro-Δ23G exhibited impaired replication, pathogenicity, and transmissibility compared to wild-type, though the co-occurring mutation T45I partially restored viral fitness. Structural analyses revealed critical conformational changes in the catalytic loop (Ile136–Val148) and b-hairpin loop (Cys22–Thr26), directly influencing inhibitor binding selectivity. These results highlight differential resistance profiles of Mpro inhibitors, supporting potential sequential or alternative use of nirmatrelvir and ensitrelvir in patients requiring prolonged antiviral treatment.
Abstract
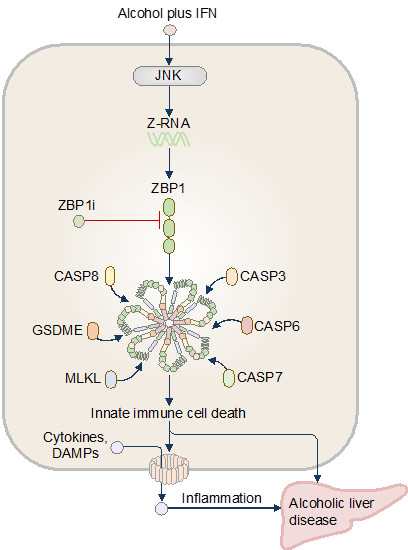
Innate Immune Recognition of DAMPs and PAMPs in Liver Disease Pathogenesis
Yeonseo Jang, Hoeun Bae, Rajendra Karki
School of Natural Sciences, Department of Biological Sciences, Seoul National University, Seoul 08826, Republic of Korea
The innate immune system is our body’s first line of defense against pathogens. Central to this system are pattern recognition receptors (PRRs) expressed by macrophages, dendritic cells, neutrophils, and natural killer (NK) cells. These receptors detect pathogen-associated molecular patterns (PAMPs) and damage/danger-associated molecular patterns (DAMPs), initiating cytokine release and programmed cell death. While essential for pathogen clearance, dysregulated innate immunity can inflict host tissue damage, fueling autoimmunity, inflammaging, and chronic inflammatory diseases. Alcohol consumption has short- and long-term impacts on physical and mental health and well-being. A combination of host and environmental factors contributes to alcohol-related diseases, but the innate immune sensors that initiate disease in response to the toxic and noxious danger signals from alcohol consumption have remained undefined. Here, we found that alcohol couples with sterile- or infection-related interferon signaling, drives innate immune cell death, cytokine release, and liver damage in humans and mice. We identified the pattern-recognition receptor ZBP1 as a sensor of ethanol and interferon. The Zα domain of ZBP1 is activated in response to Z-RNA production, driving inflammatory pyroptosis, necroptosis, apoptosis, and tissue pathology. We further identified a new small-molecule pharmacological inhibitor of ZBP1 called ZBP1i that attenuated the development of alcoholic liver disease. Our findings provide mechanistic insights into the frontline immune factors initiating and perpetuating alcohol-induced pathologies and offer therapeutic strategies for alcohol-related conditions.
Abstract
SFTSV NSs protein is a novel tick antiviral RNAi response suppressor
Mazigh Fares1#, Melanie McFarlane1#, Rhys H. Parry2#, Rozeena Arif1, Andrew T. Clarke1, Wael Kamel1, Kelsey Davies1, Lesley Bell-Sakyi3, Marine J. Petit4, Esther Schnettler5,6,7, Alfredo Castello1, Alain Kohl1,8 & Benjamin Brennan1*
1Medical Research Council–University of Glasgow Centre for Virus Research, Glasgow, G61 1QH, Scotland, UK
2School of Chemistry and Molecular Biosciences, The University of Queensland, St. Lucia 4072, Australia
3Department of Infection Biology and Microbiomes, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, L3 5RF, UK
4Microbes, Infection & Immunity, School of Biosciences, Faculty of Health and Medical Sciences, University of Surrey, Guildford, UK
5Bernhard-Nocht-Institute for Tropical Medicine, 20359 Hamburg, Germany
6German Centre for Infection Research (DZIF), Partner Site Hamburg-Luebeck-Borstel-Riems, 20359 Hamburg, Germany
7Faculty of Mathematics, Informatics and Natural Sciences, University Hamburg, 20148 Hamburg, Germany
8Liverpool School of Tropical Medicine, Centre for Neglected Tropical Diseases, Departments of Tropical Disease Biology and Vector Biology, Liverpool, L3 5QA, UK
Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne phenuivirus causing high mortality in humans. While the non-structural protein NSs is dispensable for replication in interferon-deficient mammalian cells, we demonstrate for the first time that NSs is essential for viral replication in tick cells. SFTSV infection triggers canonical Dicer-2-mediated antiviral RNA interference (RNAi) in tick cells, producing virus-derived small interfering RNAs (siRNAs) that target viral transcripts for degradation. We show that NSs functions as a viral suppressor of RNAi by selectively sequestering single-stranded RNAs derived from 22-nucleotide siRNAs, preventing their incorporation into RNA-induced silencing complexes (RISC). Complementation with a heterologous RNAi suppressor (p19 protein) partially rescues replication of NSs-deficient virus, validating NSs’ RNAi suppressive function. These findings reveal that successful tick-borne viral replication requires host-specific immune evasion strategies and establish NSs-mediated RNAi suppression as essential for SFTSV persistence in arthropod vectors.
Abstract
Lifetime dynamics of immune cells and the immune repertoire revealed by integrative single-cell analysis
Jong-Eun Park1
1 Graduate School of Medical Science and Engineering, KAIST, Daejeon, Republic of Korea
The immune system undergoes multifaceted changes with aging, including the accumulation of immunological memory and the emergence of senescence phenotypes. To comprehensively investigate immune aging, we adopted two complementary approaches. First, we constructed a large-scale collection of publicly available single-cell datasets to examine common age-associated patterns in immune cells across peripheral blood and various tissues. Using an automatic public data search process, we unbiasedly collected over 20 million single-cell transcriptomic profiles from more than 500 independent studies, which contain more than 2,000 single-cell transcriptome datasets from diverse human organs and disease states. Our analysis of this comprehensive data collection revealed that B cells and T cells exhibit distinct age-related changes, whereas myeloid cells display more heterogeneous patterns which is not determined solely by chronological aging.
In addition, we leveraged data from the Asian Immune Diversity Atlas (AIDA), which comprises healthy individuals from multiple Asian countries across diverse age groups. Through this dataset, we examined immune cell dynamics and immune repertoire alterations associated with aging. Notably, our findings indicate that clonal expansion within the immune repertoire is closely linked to T and B cell subtypes, and that immune aging trajectories may be shaped by antigen exposure. Furthermore, by integrating cellular and immune repertoire changes, we identified two major trajectories of immune aging, suggesting heterogeneity in immune aging mechanisms. These findings provide critical insights into the underlying principles of immune aging and its potential implications for age-related immune dysfunction.
Abstract
G-quadruplex regulation of cytomegalovirus infection
Jin-Hyun Ahn
Department of Microbiology, Sungkyunkwan University School of Medicine, Suwon 16419, Republic of Korea
G-quadruplexes (G4s) are non-canonical nucleic acid structures that play regulatory roles in various cellular processes, including gene expression and DNA replication. Numerous G4-forming sequences (G4 motifs) are found in herpesvirus genomes, and accumulating evidence suggests that G4 formation regulates viral gene expression and DNA replication. In human cytomegalovirus (HCMV), G4s are formed in gene promoters, and treatment with G4-binding ligands inhibits the activation of viral promoters in a promoter context-dependent manner. G4s are also found in the HCMV lytic DNA replication origin, oriLyt. G4 formation in oriLyt essential region (ER)-I is crucial for viral DNA replication. Key viral DNA replication factors bind to ER-I G4, and specific G4 ligands inhibit these interactions and suppress oriLyt activation, indicating that this oriLyt G4 acts as an essential structural element in recruiting HCMV replication factors for origin activation. G4s are also formed in the genome cleavage and packaging signals in the terminal repeat regions, and treatment of G4 ligands inhibits genome cleavage during virus infection. These studies highlight the regulatory roles of G4s in HCMV gene expression and DNA replication, suggesting that G4s may be a therapeutic target against viral infections.
- Park D, W.C. Chung, S. Gong, S. Ravichandran, G.M. Lee, M. Han, K.K. Kim, J.H. Ahn. G-quadruplex as an essential structural element in cytomegalovirus replication origin. Nat. Commun. 15:7353. 2024.
- C. Chung, S. Ravichandran, D. Park, G.M. Lee, Y.E. Kim, Y. Choi, M.J. Song, K.K. Kim, J.H. Ahn. G-quadruplexes formed by Varicella-Zoster virus reiteration sequences suppress expression of glycoprotein C and regulate viral cell-to-cell spread. PLoS Pathog. 19(1): e1011095. 2023.
Abstract
The Molecular Gatekeepers: NLRC3 and G-quadruplex Dynamics in Controlling Oncogenic Herpesvirus Latency in B Cells
Moon Jung Song
Department of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul 02841, Republic of Korea
Gammaherpesviruses (γHVs) including Epstsein-Barr virus (EBV) establish persistent infections in B lymphocytes through latency, contributing to various malignancies including lymphomas. While significant progress has been made in understanding viral persistence, the molecular mechanisms governing the establishment and maintenance of viral latency remain incompletely understood. Our research reveals two distinct molecular gatekeepers of γHV latency: the innate immune regulator NLRC3 and non-canonical DNA/RNA structures called G-quadruplexes (G4s).
Using murine gammaherpesvirus 68 (MHV-68) as a model system, we discovered that NLRC3, predominantly expressed in lymphocytes, functions as a negative regulator of viral latency. NLRC3-deficient (Nlrc3-/-) mice exhibit significantly higher latent viral loads compared to wild-type controls, despite showing no differences in acute lytic replication. Notably, γHV infection leads to reduced NLRC3 expression in B cells, and especially in EBV-infected cells, we identified that the latent membrane protein 1 (LMP1) suppresses NLRC3 expression through NF-κB. This creates a reciprocal regulatory circuit where viral proteins downregulate NLRC3 to promote latency, while NLRC3 disrupts latency by targeting the same viral protein for proteasomal degradation.
Parallel investigations into G-quadruplex structures have uncovered compelling evidence for their role in regulating γHV latency. Our computational and experimental analyses identified multiple G-quadruplex-forming sequences throughout the MHV-68 genome, with intriguing distribution patterns that suggest functional relevance during different phases of the viral life cycle. Notably, treatment with G4-stabilizing ligands significantly inhibited viral replication, suggesting these non-canonical nucleic acid structures as promising therapeutic targets. Advanced genomic approaches revealed previously uncharacterized alterations in G4 landscapes during the transition between latency and reactivation, pointing to a potentially novel regulatory mechanism governing viral persistence.
Together, these findings suggest a complex interplay of virus and host in the context of innate immune regulators and DNA/RNA secondary structures in controlling γHV latency. Our ongoing work aims to elucidate potential functional connections between G4 dynamics and cellular modulators, which may provide new insights into viral gene expression and genome maintenance during latency. This dual-pronged approach provides novel insights into molecular mechanisms of viral persistence and identifies promising targets for therapeutic intervention in γHV-associated malignancies.
Abstract
High-dimensional profiling of virus-specific T cells in virus-associated cancer and infection
Heeju Ryu
Department of Immunology, School of Medicine, Sungkyunkwan University, Suwon 440-746, Republic of Korea
Understanding the complexity of immune responses is paramount for developing effective immunotherapies and vaccines. High-dimensional profiling strategies—with mass cytometry (CyTOF) at the forefront, supported by single-cell sequencing and TCR repertoire analysis—provide the deep, single-cell resolution needed to characterize virus-specific T cell dynamics. This presentation will highlight the application of these powerful methods in two key immunological contexts.
First, we explored circulating Merkel cell polyomavirus (MCPyV)-specific CD8+ T cells in Merkel cell carcinoma patients. Our findings reveal that baseline frequencies of these tumor-specific T cells, along with distinct phenotypes characterized by CD39, cutaneous lymphocyte-associated antigen (CLA), and CD103 expression, correlate with clinical responses to anti-PD-1 therapy and overall survival. These virus-specific T cell signatures serve as potential predictive biomarkers for immunotherapy success and offer insights into tumor control mechanisms.
Second, we dissected how prior SARS-CoV-2 infection shapes T cell immunity following mRNA vaccination. Utilizing these high-dimensional tools, we demonstrated that infection-induced spike-specific CD8+ T cell memory significantly influences the magnitude and clonal composition of subsequent vaccine responses. Notably, vaccination promoted the differentiation of these memory CD8+ T cells towards a TEMRA-like phenotype and allowed for the identification of immunodominant epitopes.
Together, these studies demonstrate the power of high-dimensional immunophenotyping to reveal the functional heterogeneity and dynamic evolution of virus-specific T cells. These detailed insights are critical for identifying robust biomarkers and ultimately aid in the rational design of more effective therapeutic approaches against viral diseases and virus-associated cancers.
- Mayer-Blackwell K, Ryu H, Codd AS, et al. Cell Rep Med. 2023
- Ryu H, Bi TM, Pulliam TH, et al. Cell Rep Med. 2024
Abstract
Epithelial antigen presentation promotes lung regeneration after influenza virus infection
Subin Park, Seong Ju Cho, Jeongyong Min, Chaerim Jeong, Chang-Hyung Lee, Young-Min Kim, Sookjin Moon, Ohbin Kwon, Yoojin Jin, Yoon Ha Choi, Byunghee Kang, Kwang Soon Kim, Tae Young Roh, Jinwook Choi, Heetak Lee, Joo-Hyeon Lee, Jong Kyoung Kim, Bon-Kyoung Koo, Seung-Woo Lee
The alveolar epithelium is essential component for lung defense and homeostasis. It consists mainly of two cell types: alveolar type 1 (AT1) and type 2 (AT2) cells. AT1 cells are large squamous cells that cover more than 95% of the alveolar surface area, mediating air-blood gas exchange. AT2 cells, on the other hand, are organelles-rich cuboidal cells that controls surface tension of alveoli through surfactant. In addition, AT2 cells play a central role as alveolar epithelial progenitors, proliferating and differentiating into AT1 cells to restore gas exchange and tissue integrity. Disorders such as long COVID highlight the challenges of incomplete regeneration, often resulting in fibrosis and impaired breathing. The regenerative capacity of AT2 cells is finely tuned through various environmental factors. During recovery, AT2 self-renewal is supported by Treg- and fibroblast-derived amphiregulin, while inflammatory cues such as IL-1β and TNFα prime them to differentiate into AT1 cells once inflammation subsides. Notably, AT2 cells exhibit uncanonical MHC-II expression, a molecule typically involved in antigen presentation to CD4 T cells. However, the potential of MHC-II during the AT2-mediatd alveolar regeneration, as well as the inducer of MHC-II in AT2 cells, are limitedly understood. Using single-cell transcriptomics and imaging in developing mouse lungs and organoids, we found that fibroblast-driven niche signals induce MHC-II expression in AT2 cells during postnatal maturation. Following influenza infection, AT2 cells secreted chemokines and formed immune synapses with CD4 T cells, functioning as atypical APCs. While loss of MHC-II in AT2 cells did not compromise overall antiviral immunity, it disrupted epithelial repair, reducing AT1 cell differentiation, resulting in post-acute sequelae after IAV infection. Together, our findings uncover a non-canonical role of AT2 cells as epithelial antigen-presenting progenitors, through which effective alveolar repair is mediated after injury.
Abstract
T cell differentiation landscapes after COVID-19 vaccination
Min Kyung Jung
The Center for Viral Immunology, Korea Virus Research Institute, Institute for Basic Science, Daejeon, Korea, 34126
Memory T cells are central to sustained protection against SARS-CoV-2, yet their durability and quality are shaped by the nature of immune exposure. Here, we longitudinally profiled SARS-CoV-2-specific memory T cell responses induced by vaccination and natural infection. Vaccination elicited diverse T cell subsets, including stem cell-like memory T cells (TSCM), which were maintained over extended periods and further boosted by breakthrough infection. Different vaccine platforms and diverse antigen exposures influenced the balance of subset distribution, functionality, and proliferative potential. Importantly, breakthrough infection broadly enhanced memory T cell responses across exposure histories. Collectively, these findings highlight how vaccine design, antigen breadth, and post-vaccination infection shape T cell differentiation landscapes, with implications for optimizing long-lived cellular immunity in the endemic era.
Abstract
Metabolic Control of Humoral Immunity
Jiyeon Kim, Hyun-Uk Yeo, Yeonseok Chung
Leader Research Center for Immunometabolism, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea
Plasma cells are essential for host defense, producing neutralizing antibodies in response to infection or vaccination, some of which persist in the bone marrow for a lifetime. Despite being the body’s most secretory cells, the mechanisms governing their antibody production and longevity are not fully understood. In particular, how plasma cells manage chronic ER stress without inducing cell death remains unclear. Here, we employed lipidomic and transcriptomic analyses to identify a unique lipid metabolism program in plasma cells, distinct from that of activated B cells, characterized by specific expression of liver X receptor (LXR) exclusively in plasma cells. LXR-deficient plasma cells are more prone to cell death. During plasma cell differentiation from activated B cells, IRE1α activation induces LXR expression. LXR exerts a protective role by selectively inhibiting the PERK-ATF4-CHOP pathway of ER stress, thereby preventing plasma cell apoptosis. Moreover, LXR-mediated upregulation of SCD is critical for maintaining monounsaturated fatty acid (MUFA) levels, which protect plasma cells from ATF4-driven apoptosis. Our findings uncover a previously unrecognized role for LXR as a key regulator of plasma cell longevity through the modulation of lipid metabolism.
Abstract
Distinct Localization of Cytotoxic and Cytokine-producing CD8+ T Cells in the Liver during OX40-mediated Virus Inactivation
Keigo Kawashima, Masanori Isogawa
Department of Virology II, National Institute of Infectious Diseases, Japan Institute for Health Security, Tokyo 162-8640, Japan
Hepatitis B virus (HBV)-specific CD8+ T cells terminate HBV infection by exerting cytotoxicity and producing antiviral cytokines, but these effector functions are suppressed during persistent infections. It has not been investigated whether microanatomical characteristics in the liver influence the effector functions. We here demonstrate that OX40 activation of HBV-specific CD8 T cells during intrahepatic priming transiently induces effector function and suppresses HBV transcription. Remarkably, the viral transcription remained inactivated almost half a year despite the presence of template DNA and the absence of continuous T cell activation. Spatial transcriptomic analysis of HBV-specific CD8 T cells in the liver revealed that dysfunctional CD8+ T cells were localized in the periportal area, although HBV transcription was more active in the centrilobular area. In contrast, OX40-activated CD8+ T cells exhibited a more homogenous distribution throughout the liver. Notably, genes associated with T cell cytotoxicity colocalized with Cd8a throughout the OX40-activated liver, while the region displaying IFNγ transcription was sparse and predominantly in centrilobular. These results reveal the existence of distinct microanatomical niches for dysfunctional, cytotoxic, and cytokine-producing T cells.
- Kawashima, K, Onishi M, Kanto T, Iannacone M, Saito S, Nakajima A, Suzuki Y, Tanaka Y, Isogawa M,
- Andreata F, Laura C, Ravà M, Krueger CC, Ficht X, Kawashima K, Beccaria CG, Moalli F, Partini B, Fumagalli V, Nosetto G, Di Lucia P, Montali I, Garcia-Manteiga JM, Bono EB, Giustini L, Perucchini C, Venzin V, Ranucci S, Inverso D, De Giovanni M, Genua M, Ostuni R, Lugli E,Isogawa M, Ferrari C, Boni C, Fisicaro P, Guidotti LG, Iannacone M. Therapeutic potential of co-signaling receptor modulation in hepatitis B. Cell. 2024 Jul 25;187(15):4078-4094.e21. doi: 10.1016/j.cell.2024.05.038
Abstract
Latent MCMV Infection and MASH
You-Me Kim
Graduate School of Medical Science and Engineering, KAIST, Daejeon 34051, Republic of Korea
Cytomegalovirus (CMV) is a highly prevalent herpesvirus, with seropositivity exceeding 80% worldwide. While latent infection remains clinically silent in immunocompetent individuals, accumulating evidence suggests that CMV-seropositive individuals may be predisposed to chronic inflammatory diseases. However, the underlying mechanisms remain poorly defined. Given that latent CMV infection induces profound and lasting alterations of the immune system including memory inflation and senescence of CD8 T cells, we investigated the contribution of latent murine CMV (MCMV) infection to the pathogenesis of metabolic-associated steatohepatitis (MASH). In a high-fat diet (HFD) model, mice with prior latent MCMV infection developed significantly more severe steatohepatitis than HFD-only controls, as evidenced by exacerbated liver inflammation, fibrosis, and elevated serum ALT levels. Immunophenotyping revealed a striking expansion of hepatic CD8 T cells in MCMV+HFD mice, with enhanced expression of IFN-γ and granzyme A, indicating augmented effector function. Single-cell RNA sequencing demonstrated that the expanded CD8 T cells in MCMV+HFD mice displayed distinct transcriptional profiles compared to the pathogenic CXCR6⁺PD-1⁺CD8 T cells typically induced by HFD alone. Notably, MCMV-specific CD8 T cells exhibited elevated expression of IL-15 and IL-18 receptors, as well as NK receptors, suggesting increased potential for bystander activation. Functional assays further showed that these MCMV-specific CD8 T cells were highly responsive to IL-15, upregulating NKG2D and effector molecules, and acquiring heightened NK-like cytotoxicity. Together, these findings demonstrate that latent MCMV infection exacerbates MASH progression by reshaping the hepatic CD8 T cell compartment toward enhanced responsiveness and cytotoxicity. This work highlights the need to consider latent CMV infection as an important immunological factor in chronic metabolic liver disease.
Abstract
The many faces of responding T cells during checkpoint therapy between efficacy and toxicity: uncovering targetable mechanisms of reprogramming in time and space
The mechanisms underlying the efficacy and toxicity of anti-programmed cell death protein 1 (PD-1) and anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) therapy are incompletely understood. First, by studying the programming of responding PD-1+CD8+ T (Tresp) cell populations from patients with advanced melanoma, we identified differential programming of Tresp cells in response to combination therapy, from an exhausted toward a more cytotoxic effector program. This effect does not occur with anti-PD-1 monotherapy. Single-cell transcriptome and T cell receptor repertoire analysis was used to identify altered effector programming of expanding PD-1+CD8+ T cell clones with distinct regulon usage, STAT1 and STAT3 utilization and antitumor specificity connected to interleukin (IL)-21 signaling in combination and anti-CTLA-4 monotherapy. Therapeutic efficacy of CTLA-4 blockade was lost in B16F10 melanoma models with either Il21r- deficiency or anti-IL-21 receptor blockade. Together, these results show how IL-21 signaling to TResp is critical for anti-CTLA-4-based checkpoint therapies and highlight major signaling differences to anti-PD-1 monotherapy. Nevertheless, checkpoint therapy induces significant and potential life-threatening immune-mediated side-effects, such as the liver. By spatial single-cell and transcriptomic analysis of the inflamed livers of checkpoint therapy induced-hepatitis (ICI-Hep) and spontaneous autoimmune hepatitis, we identify responding T cells as hallmarks of pathological liver inflammation and identify distinct interaction partners and therapeutically targetable signaling pathways. The presentation highlights differential pathways mediating the reprogramming of exhausted T cells downstream of checkpoint therapy.
Registration
Registration Period
October 13th – December 7th
Registration Fee
Student: 70,000 KRW
Regular: 100,000 KRW
Notice
Registration will be open soon.
Please try later.
Venue
2F, Grand Ballroom, Daejeon Convention Center 1 (DCC 1), Daejeon, South Korea
107 Expo-ro, Yuseong-gu, Daejeon, South Korea, 34125