| Title of announcement | Cellular regulator of mRNA vaccine revealed... offering new therapeutic options | ||||
|---|---|---|---|---|---|
| Business forms | Expiration date for bidding | ||||
| Department in charge | 전체관리자 | Registration Date | 2025-04-04 | Hits | 55 |
| att. |
 Figure 1.jpg
Figure 1.jpg
 Figure 2.jpg
Figure 2.jpg
|
||||
|
|
|||||
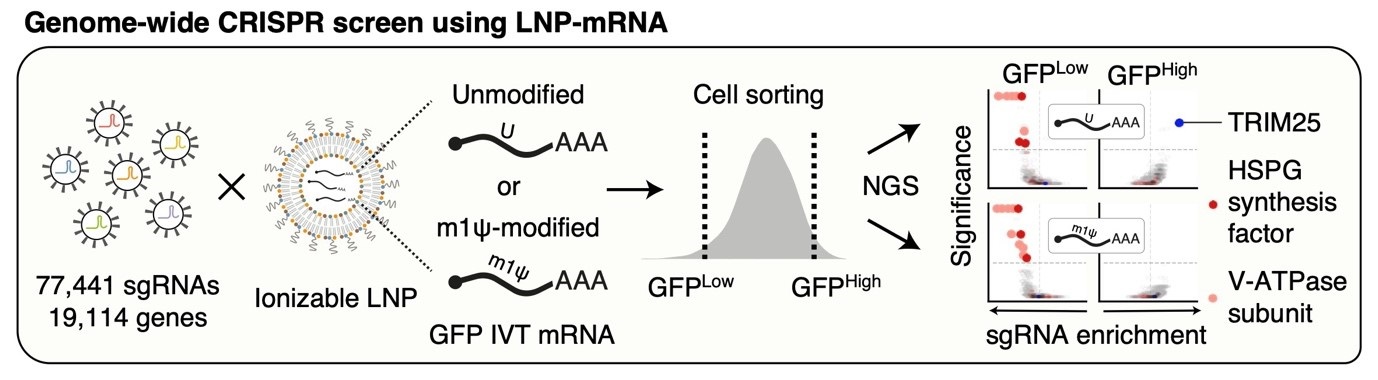
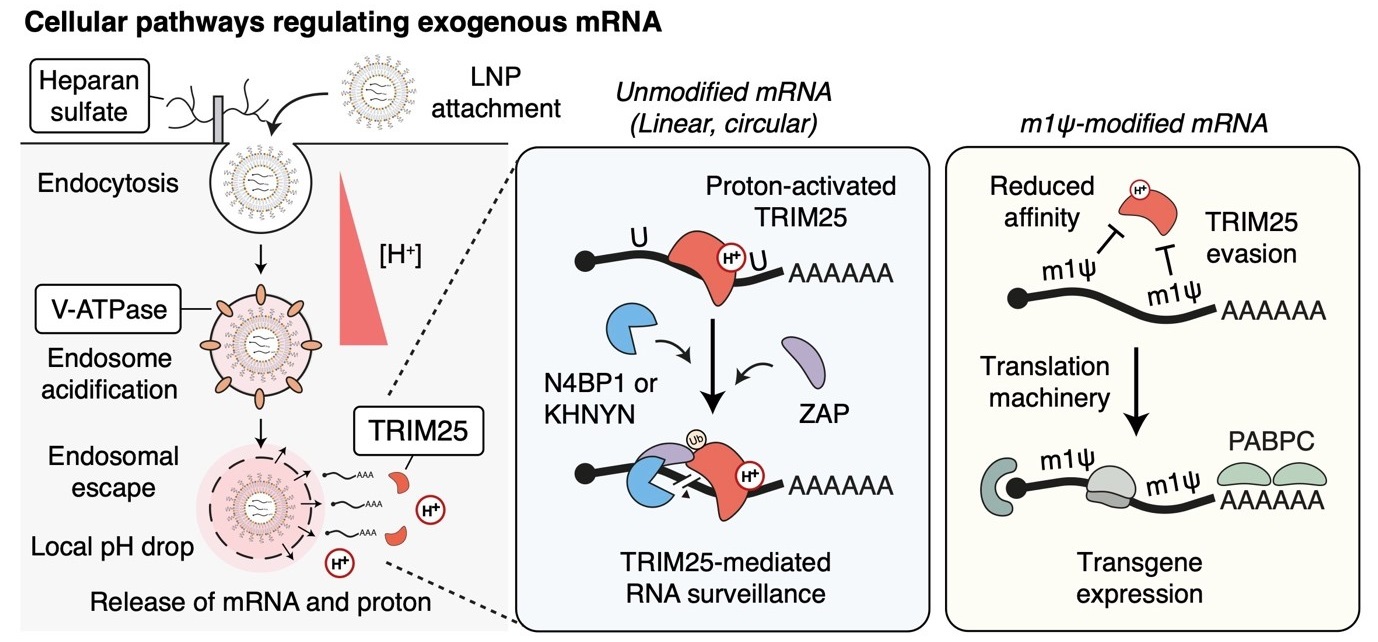
Cellular regulator of mRNA vaccine revealed... offering new therapeutic options- Cellular factors that regulate the delivery and stability of mRNA vaccines in the body were revealed, proposing a new paradigm for mRNA therapeutics – A team of researchers led by Dr. KIM V. Narry, director of the Center for RNA Research at the Institute for Basic Science (IBS), has uncovered a key cellular mechanism that affects the function of mRNA vaccines and therapeutics. Their study, recently published in Science, provides the first comprehensive understanding of how mRNA vaccines are delivered, processed, and degraded within cells—a breakthrough that could pave the way for more effective vaccines and RNA-based treatments. Messenger RNA (mRNA) is the genetic blueprint that tells cells how to produce proteins. It plays a vital role in mRNA vaccines, such as those used for COVID-19, and is also a promising tool for treating diseases like cancer and genetic disorders. When foreign mRNA, such as those in mRNA vaccines enters cells, it must evade the body's natural defense mechanisms to be effective. However, the detailed mechanisms by which mRNA is regulated inside cells have remained largely unknown. The research team employed CRISPR-based knockout screening to identify the cellular factors involved in the delivery of mRNA into cells. This approach, using a CRISPR library targeting 19,114 genes, revealed three key factors that facilitate the cellular uptake or surveillance of exogenous mRNAs. - First, the team discovered that heparan sulfate (HSPG), a sulfated glycoprotein on the cell surface, plays a crucial role in attracting LNPs and facilitating mRNA entry into the cell. - Second, they identified V-ATPase, a proton pump at the endosome, which acidifies the vesicle and causes LNPs to become positively charged, enabling them to temporarily disrupt the endosomal membrane and release the mRNA into the cytoplasm to be expressed. - Lastly, the study uncovered the role of TRIM25, a protein involved in the cellular defense mechanism. TRIM25 binds to and induces the rapid degradation of exogenous mRNAs, preventing their function. So how do the mRNA vaccines evade this cellular defense? A key finding of the study was that mRNA molecules containing a special modification called N1-methylpseudouridine (m1Ψ)—which was awarded the 2023 Nobel Prize in Physiology or Medicine—can evade TRIM25 detection. This modification prevents TRIM25 from binding to mRNA, enhancing the stability and effectiveness of mRNA vaccines. This discovery not only explains how mRNA vaccines evade cellular surveillance mechanisms but also emphasizes the importance of this modification in enhancing the therapeutic potential of mRNA-based treatments. Additionally, the research highlighted the critical role of proton ions in this process. When the LNPs rupture the endosomal membrane, proton ions are released into the cytoplasm, which activates TRIM25. These proton ions act as a signal that alerts the cell to the invading foreign RNA, which in turn triggers a defense response. This is the first study to demonstrate that proton ions serve as immune signaling molecules, providing new insights into how cells protect themselves from foreign RNA. Dr. KIM V. Narry emphasized the importance of understanding these processes, stating, "Understanding how cells respond to mRNA vaccines is key to improving mRNA therapeutics. To develop effective RNA treatments, we need to find ways to bypass the cellular defense mechanisms and harness the endosomal system effectively." This research, published in Science on April 3rd, not only paves the way for more efficient mRNA vaccine delivery but also offers a framework for future development of RNA-based therapies. The findings underscore the critical importance of early intervention and provide new directions for developing more effective treatments for a variety of diseases.
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS)
|
|||||