| 공고명 | Innovative Microscopy Technique Reveals Secrets of Lipid Synthesis Inside Cells | ||||
|---|---|---|---|---|---|
| 업무형태 | 입찰마감일 | ||||
| 담당처 | 전체관리자 | 등록일 | 2024-01-24 | 조회 | 148 |
| 첨부 | |||||
|
|
|||||
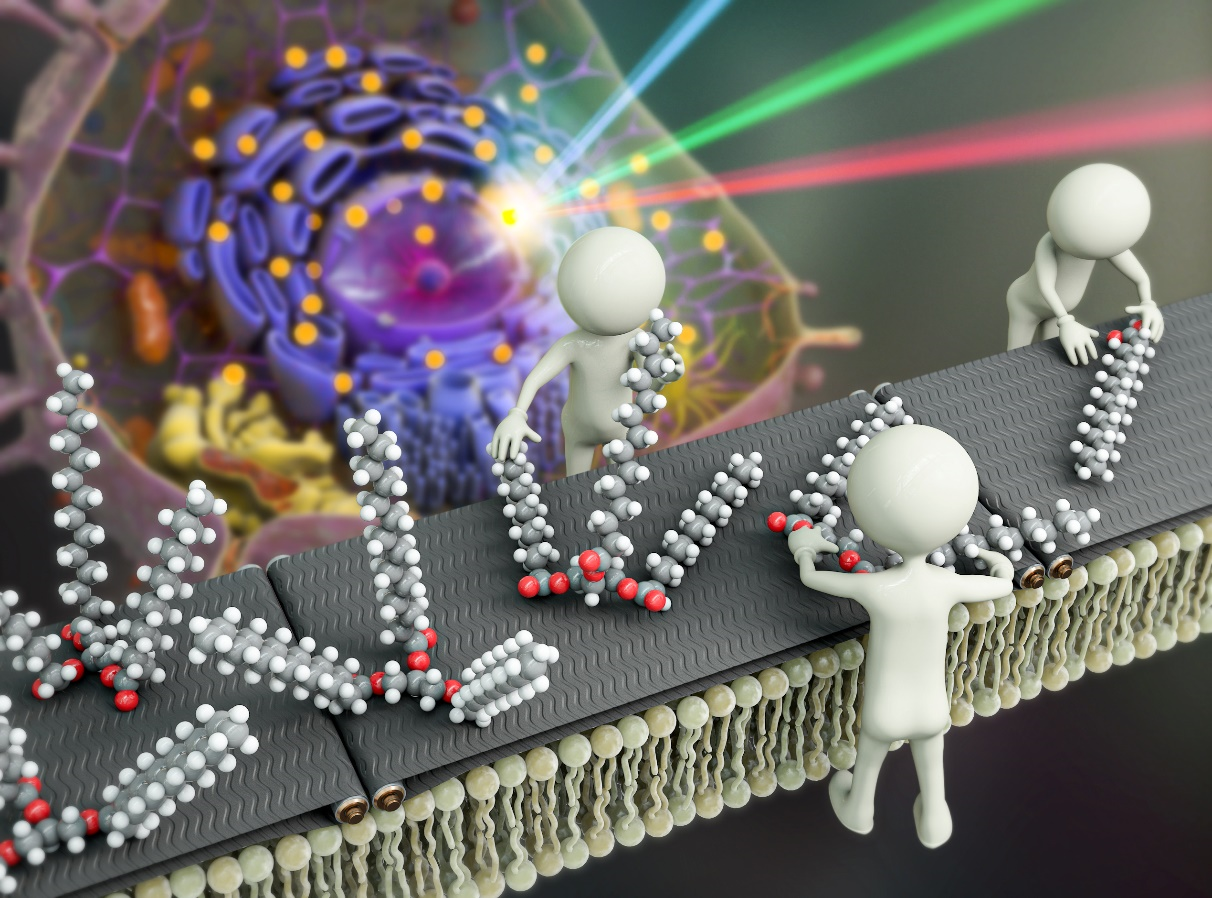
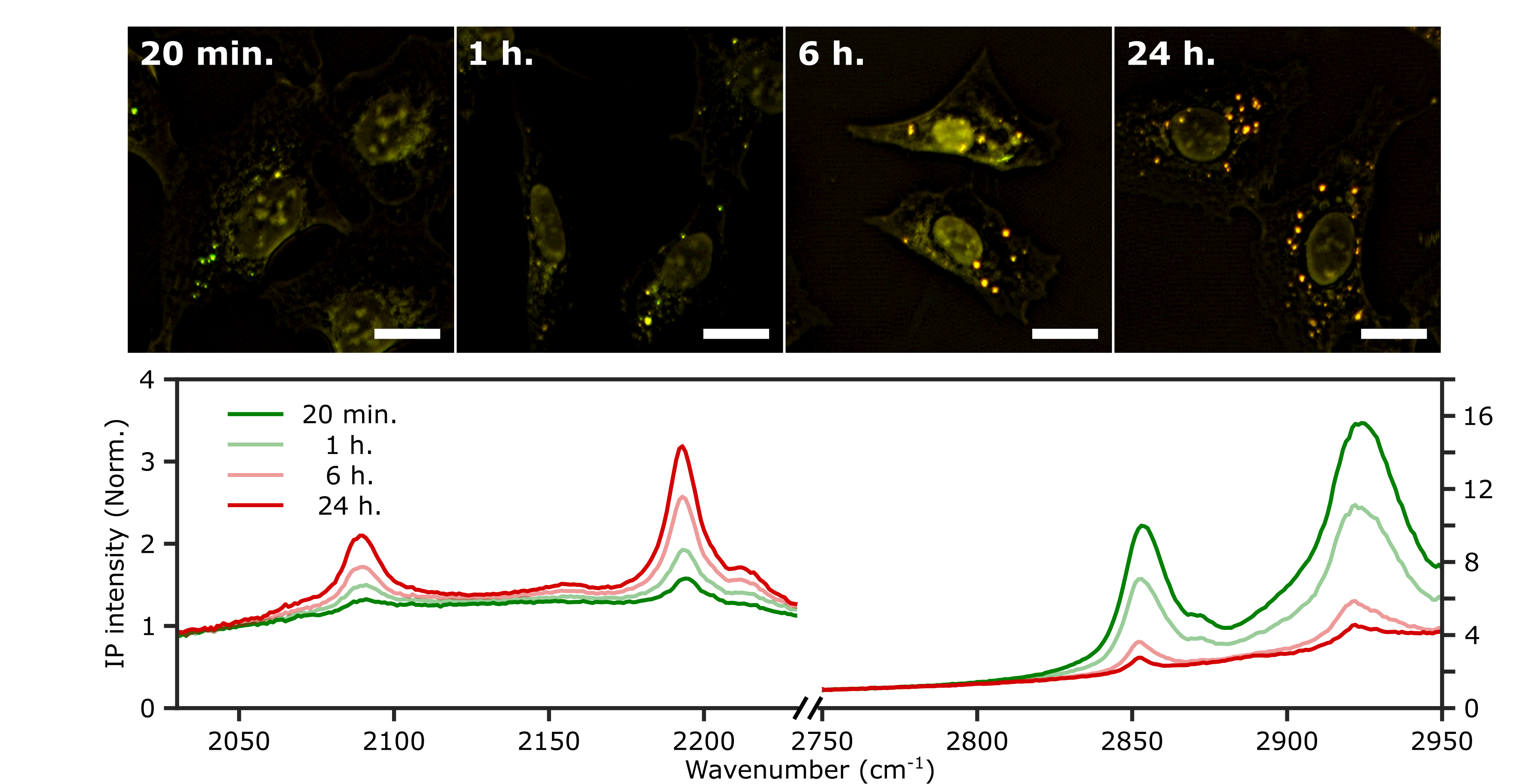
Innovative Microscopy Technique Reveals Secrets of Lipid Synthesis Inside Cells- Two-Color Infrared Photothermal Microscopy (2C-IPM) Opens New Avenues for Long-Term Study of Lipid Metabolism in Living Cells - South Korean researchers led by Director CHO Minhaeng at the IBS Center for Molecular Spectroscopy and Dynamics (IBS CMSD) made a pivotal discovery in the field of cellular microscopy. The team has successfully developed Two-Color Infrared Photothermal Microscopy (2C-IPM), a novel technology designed to investigate neutral lipids within lipid droplets of living cells. This new microscopy can be used with isotope labeling, which allows for the detailed monitoring of neutral lipid synthesis within individual lipid droplets. Lipid droplets (LDs) are structure that consists of pockets of neutral lipids (triglycerides) encapsulated in a monolayer of phospholipids. They have long been thought of as relatively uninteresting organelles, whose only purpose is to store excess energy in the form of neutral lipids. However, recent studies indicate these droplets actually are dynamic players in various cellular metabolic activities. It was found they are actively involved in regulating lipid toxicity and cell communication, as well as their correlation with prevalent diseases like obesity and non-alcoholic fatty liver disease. Understanding the functions of LDs is thus imperative for the diagnosis and treatment of these conditions. Researchers have traditionally stained the cells with lipophilic dyes and employed fluorescence microscopy to study LDs within cells. However, this method has several significant limitations. First is photobleaching of the dyes, which restricts the observation duration of the LDs to very short time windows. Another limitation is the fluorescent dyes themselves. The currently used fluorescent dyes simply target the hydrophobic environment within LDs, utilizing unspecified binding mechanisms. As such, they are incapable of accurately analyzing the composition and quantity of neutral lipids. Unlike previous methods that relied on fluorescent microscopy, the new 2C-IPM technology uses an infrared (IR) spectroscopic method and does not require the use of fluorescent dyes. The new method monitors neutral lipids within LDs directly by detecting changes in IR absorbance. Importantly, this advantage allows researchers to observe the synthesis of neutral lipids within individual LDs in living cells over a long period. Employing the newly developed method, the research team studied the synthesis of neutral lipids in cells when they were exposed to excess fatty acids. The researchers were able to distinguish freshly synthesized neutral lipids from pre-existing neutral lipids within cells by subjecting deuterium-labeled fatty acids, which have distinct spectroscopic properties from non-deuterated forms. The analysis results verified excess fatty acids caused lipid toxicity, and cells respond by increasing the synthesis of neutral lipids. The lead author, researcher PARK Chanjong, remarked, "This study serves as a fundamental example demonstrating the possibility of long-term research on lipid droplets and internal neutral lipids in living cells. The analytical method established in this research can be applied to the diagnosis and treatment of diseases closely associated with lipid metabolism, such as non-alcoholic fatty liver disease." Director CHO Minhaeng commented, "By successfully observing the process of neutral lipid synthesis in living cells, we have laid a new foundation for studying the functions of lipid droplets within cells at the molecular level. This method is expected to be widely used in the investigation of various cellular metabolic phenomena."
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS)
|
|||||