주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
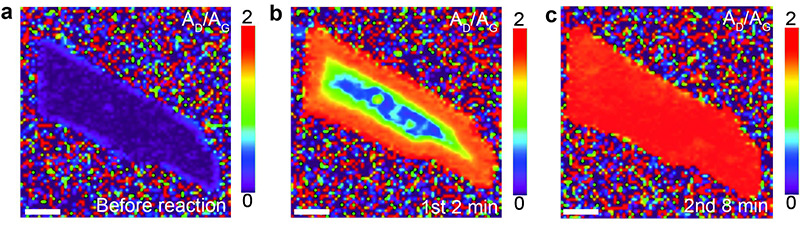
Adding Hydrogen to Graphene- IBS researchers report fundamental study of how graphene is hydrogenated - Adding hydrogen to graphene could improve its future applicability in the semiconductor industry, when silicon leaves off. Researchers at the Center for Multidimensional Carbon Materials (CMCM) (http://cmcm.ibs.re.kr/html/cmcm_en/), within the Institute for Basic Science (IBS) have recently gained further insight into this chemical reaction. Published in Journal of the American Chemical Society, these findings extend the knowledge of the fundamental chemistry of graphene and bring scientists perhaps closer to realizing new graphene-based materials. Understanding how graphene can chemically react with a variety of chemicals will increase its utility. Indeed, graphene has superior conductivity properties, but it cannot be directly used as an alternative to silicon in semiconductor electronics because it does not have a bandgap, that is, its electrons can move without climbing any energy barrier. Hydrogenation of graphene opens a bandgap in graphene, so that it might serve as a semiconductor component in new devices. While other reports describe the hydrogenation of bulk materials, this study focuses on hydrogenation of single and few-layers thick graphene. IBS scientists used a reaction based on lithium dissolved in ammonia, called the "Birch-type reaction", to introduce hydrogen onto graphene through the formation of C-H bonds. The research team discovered that hydrogenation proceeds rapidly over the entire surface of single-layer graphene, while it proceeds slowly and from the edges in few-layer graphene. They also showed that defects or edges are actually necessary for the reaction to occur under the conditions used, because pristine graphene with the edges covered in gold does not undergo hydrogenation.
Using bilayer and trilayer graphene, IBS scientists also discovered that the reagents can pass between the layers, and hydrogenate each layer equally well. Finally, the scientists found that the hydrogenation significantly changed the optical and electric properties of the graphene. "A primary goal of our Center is to undertake fundamental studies about reactions involving carbon materials. By building a deep understanding of the chemistry of single-layer graphene and a few layer graphene, I am confident that many new applications of chemically functionalized graphenes could be possible, in electronics, photonics, optoelectronics, sensors, composites, and other areas," notes Rodney Ruoff, corresponding author of this paper, CMCM director, and UNIST Distinguished Professor at the Ulsan National Institute of Science and Technology (UNIST). Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Multidimensional Carbon MaterialsPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20