주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
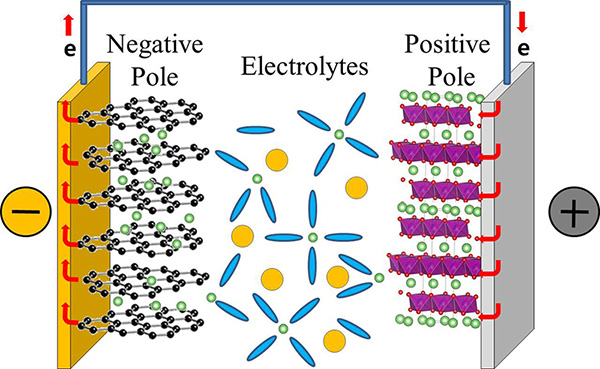
Clarifying How Lithium Ions Ferry Around in Rechargeable Batteries- IBS scientists observe the real-time ultrafast bonding of lithium ions with solvents, in the same process that happens during charging and discharging of lithium-ion batteries, and conclude that a new theory is needed - Although most of our electronic devices, like mobile phones, laptops, and electric vehicles use lithium-ion rechargeable batteries, what is going on inside them is not fully understood. Researchers from the Center for Molecular Spectroscopy and Dynamics, within the Institute for Basic Science (IBS) succeeded in observing in real-time the ultrafast dynamics of lithium ions with femtosecond time resolution (1/1,000,000,000,000,000 of a second). These findings help to explain what happens during the process of charging and discharging; a cornerstone for advanced batteries with better performance. Published in Nature Communications, this study reveals the interactions between lithium ions and electrolytes, which are organic molecules that surround the lithium ions and conduct electricity, and were able to conclude that the existing theory on ion diffusion in lithium ion rechargeable batteries is not completely correct. In a typical commercial lithium-ion rechargeable battery, lithium ions dissolved in electrolytes move from the positive to the negative pole of the battery when the battery is charging. They migrate in the opposite direction when the battery is in use. The lithium-ion mobility determines the performance of a lithium-ion battery, and determines how rapidly they can charge and discharge.
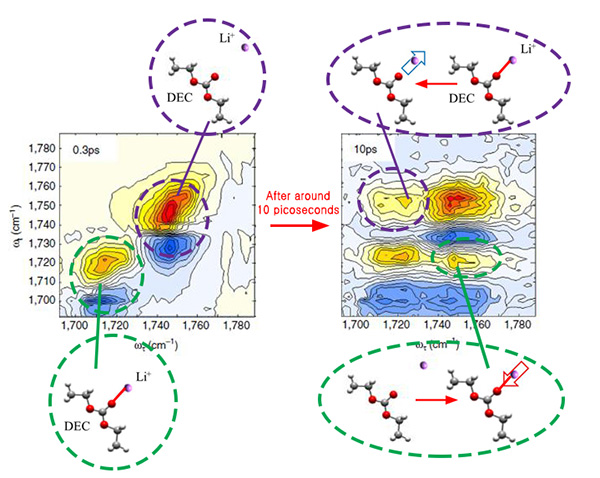
Lithium ions, however, do not migrate alone, they are surrounded by electrolytes that facilitate the journey from one pole to the other. Currently, the electrolytes in lithium-ion batteries are typically composed of a mixture of ethylene carbonate (EC), dimethyl carbonate (DMC), and diethyl carbonate (DEC) in equal concentration. It is believed that lithium ions associate mainly with EC, forming the so-called "solvation shell" or "solvation sheath", while DMC and DEC are just enhancing the movement of these shells between the batteries' poles, much like a lubricant. However, while most previous studies focused on the static properties of the bond between electrolytes and lithium ions, this study clarifies the dynamics of the bonding. Like in a motion picture, where a series of still images displayed rapidly one after the other create the effect of movement, IBS scientists took quick photos to analyze the formation and breaking of these bonds. However, while movies are typically filmed and displayed at 24 still images per seconds, these measurement images were taken at time intervals of just femtoseconds. Thanks to a tool called two-dimensional infrared spectroscopy, the team measured how lithium ions bind to the oxygen atoms of DEC and found that these bonds break and form in a matter of 2-17 picoseconds (1/1,000,000,000,000 of a second). The timescale is similar for DMC. This means that DMC and DEC are more than just "lubricants", they are also part of the solvation shell together with EC and may play an active role in transporting lithium ions to the battery's pole.
"It was believed that EC makes a rigid shell around lithium ions during the migration between electrodes. However, this study shows that the solvent shell is not that rigid, it is constantly restructured during the ion transport," explains Professor CHO Minhaeng. And concludes: "For this reason, revising the existing lithium-ion diffusion theory is inevitable." The research team is working on a follow-up study to establish a new theory of the lithium-ion diffusion process and it is building a new ultra-high-speed laser spectroscopy instrument that can observe the chemical reaction as well as film videos of it. Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Molecular Spectroscopy and DynamicsPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20