주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
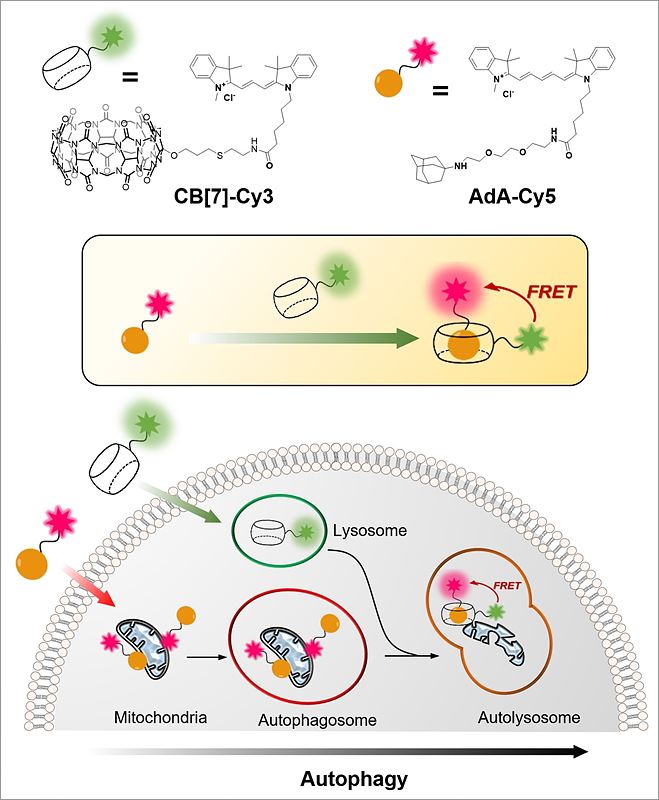
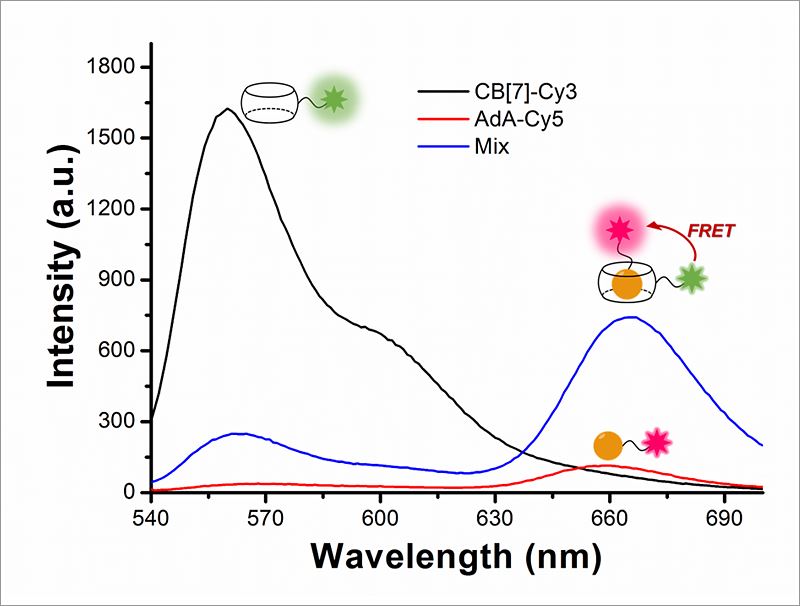
Cellular Recycling Caught In the ActNew bioimaging technology visualizes cellular recycling, technically known as mitophagy, A team of researchers at the Center for Self-assembly and Complexity, within the Institute for Basic Science (IBS) have observed a normal physiological process, called "self-eating", that cells use to recycle their components. They developed an accurate technique that visualizes how mitochondria, cells’ energy factories, are fused with lysosomes, cells’ recycling machines, in order to get material destroyed and recycled. Since irregularities in this mechanism can lead to Alzheimer's, Parkinson's, and other complications, this research could help future advances in degenerative brain disease diagnosis and drug development. The study was published in Angewandte Chemie International Edition. The name "autophagy" comes from the Greek language and means "self-eating". As weird as it is sounds, it is an indispensable process used to clean cells from damaged organelles and proteins. Inside lysosomes, old cellular parts are broken down to their building blocks, which are employed to build new ones. Recently, autophagy became a very active field of study and was at the center of Yoshinori Ohsumi’s Nobel Prize in Physiology or Medicine in 2016. One example of cellular organelle that must be periodically recycled is the mitochondrion. The careful regulation of this process is essential, and cells that do not divide regularly, like nerve cells, are particularly vulnerable. If cells accumulate defective mitochondria, they can damage themselves. This occurs, for example, in Alzheimer's and Parkinson's diseases, where build-up of damaged mitochondria and aggregated proteins leads to neuronal death. It is known that autophagy (or mitophagy, in the specific case of mitochondria) occurs by fusion of two different organelles, in this case: mitochondria and lysosomes. However, observing the behavior and fusion of mitochondria and lysosomes inside the cells has been challenging. To date, most of these studies rely on fluorescent proteins attached to one organelle, which allows the scientists to observe only one organelle at a time. Moreover, fluorescent proteins themselves are degraded during autophagy, making it difficult to accurately study the mechanism. IBS scientists at POSTECH devised a quantitative procedure to visualize both mitochondria and lysosomes over time. The relatively simple and inexpensive technique is more accurate than the current ones. It uses hollow barrel-shaped synthetic molecules, known as cucurbituril (CB[7]), that binds with exceptionally high binding strength to a molecule called adamantylamine (AdA) and cannot be degraded by the lysosomes. CB[7] was decorated with a fluorescent dye (Cy3), while AdA with another dye (Cy5). Initially, CB[7]-Cy3 enters the lysosomes and Ada-Cy5 the mitochondria, and then, when the two organelles fuse in the recycling process, CB[7]-Cy3 and Ada-Cy5 bind together. In this way, the research team followed the behavior of different organelles and observed the mitophagy process occurring in live cells. Moreover, the experiments showed that the two compounds are not toxic for the cells below a 800 nanomolar dose.
"The most challenging part of the experiment was the choice of the chemicals. We chose chemicals with the right hydrophilicity, charge, and molecular size to be selectively incorporated into mitochondria and lysosomes," explains PARK Kyeng Min, one of the corresponding authors of the study. In more technical terms, the novelty of this paper relies on the application of fluorescence resonance energy transfer (FRET) to the study of autophagy. FRET is a distance-dependent transfer of energy between two different fluorescent dyes; in this case Cy3 and Cy5. When the two are close enough, Cy3 donates energy to Cy5. The scientists capture the meeting of the two dyes as the transfer of energy from Cy3 to Cy5 leads to a reduction in Cy3 fluorescence intensity in favor of an increase in Cy5 emission intensity.
In the future, a similar technique could be used to study autophagy processes that involve other cellular organelles, like the protein factories Golgi apparatus and endoplasmic reticulum. Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Self-assembly and ComplexityPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20