주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
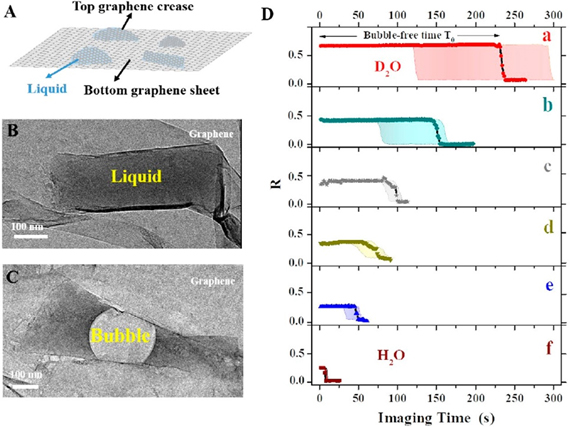
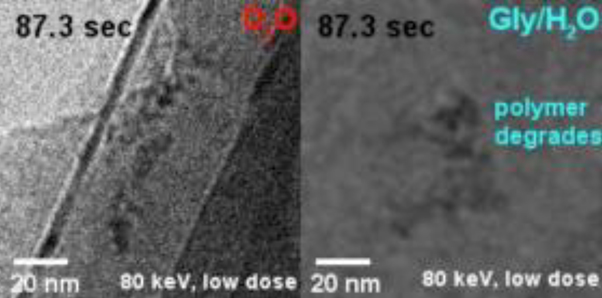
Sunscreen for Dancing Molecules- Deuterated water (D2O) prolongs the stability of samples under an electron microscope, allowing scientists to observe the movements of the nanoworld for longer – Since life is mostly based on water, our molecules are moving, vibrating and somersaulting in a liquid environment. But electron microscopy, a superb instrument to study a static version of the nanoworld, has been almost impossible to use to see moving molecules because the incident electron beam damages the samples. Scientists at the Center for Soft and Living Matter, within the Institute for Basic Science (IBS), now report a big improvement. This study, published in ACS Nano, is the first to use heavy water (D2O) - a form of water that contains deuterium (D) instead of hydrogen - in the field of transmission electron microscopy (TEM). This approach significantly delays sample damage, which is one of the major impediments for broader application of liquid-phase TEM to fragile biological samples. In electron microscopy, electrons shot against the sample have a much shorter wavelength than light, so they are better suited to provide information about single molecules. On the other hand, the electron beam is violently strong and risks damaging the specimen due to its high energy, which generates an electric charge and breaks the chemical bonds. IBS researchers used a tiny pocket filled with liquid sandwiched between atomically-thin sheets of graphene, within which the sample molecules are free to move and are protected from electrical charging, and tested several types of liquids to find the one that preserves the sample longer. “In contrast to the common approach of reducing the energy of the electron beam to delay sample damage, we focused on tuning the environment; the water in which the molecules of interest are dissolved,” says Huan Wang, co-author of the study. IBS scientists have shown that use of heavy water has several advantages over competing methods. D2O delays most effectively not only the formation of gas bubbles, but also structural damage of individual polymer molecules. Compared to H2O, D2O has one more neutron, which means that it is heavier, thus more difficult to dissociate into radicals, and less reactive in the subsequent damaging process. “Heavy water outperforms the competing methods by a factor of two to five at least,” specified Kandula Hima Nagamanasa, co-author of the study. “Since bubble formation is delayed, and the molecules were visible for twice as long.”
An equally important advantage is that D2O is a harmless sunscreen. The sample, a polymer of polystyrene sulfonate in this case, showed the same pattern of dynamics and similar contrast in D2O and in water. “In the future, we plan to extend this study to more complex macromolecules, like DNA and proteins,” explained Steve Granick, director of the IBS center and corresponding author of the study. “Moreover, the study opens avenues to observe long-term phenomena in other related microscopy techniques, like cryoEM (cryogenic electron microscopy), and to get more statistical information about complex phenomena, like self-assembly of single molecules into more complex biological structures.” Letizia Diamante, Burdette Choi Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Soft and Living MatterPublication Repository |
|||
|
|
| Next | |
|---|---|
| Next |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20