주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
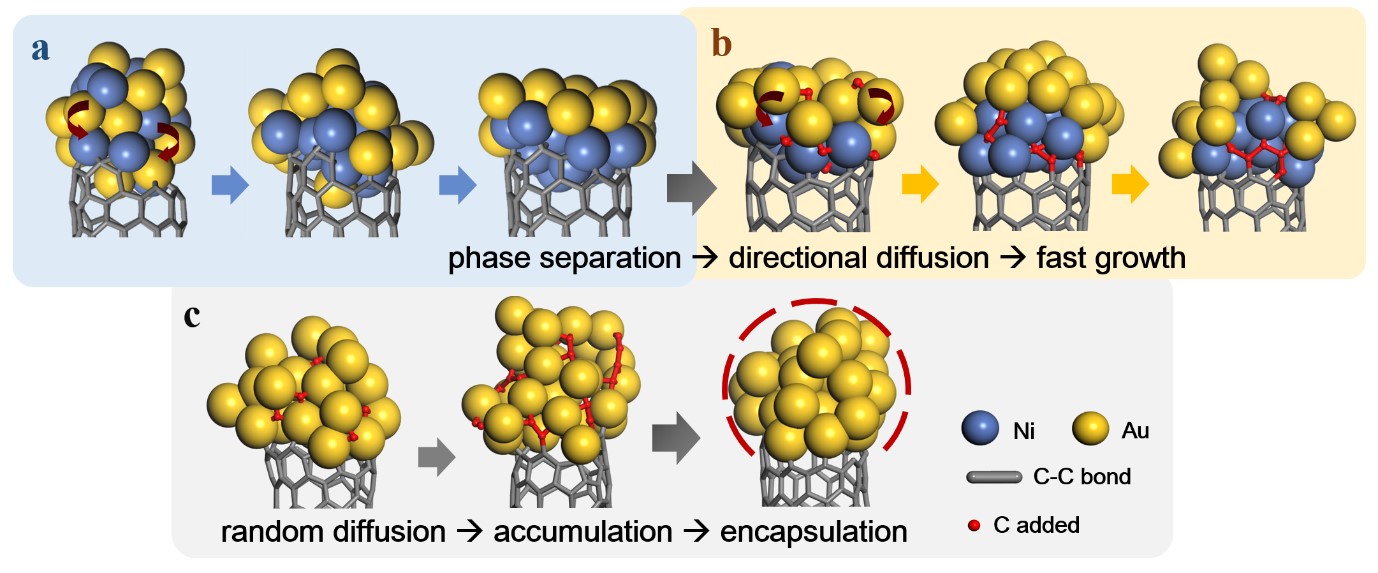
Why are alloy metal nanoparticles better than monometallic ones for carbon nanotubes growth?- Revealing a long-term mystery of why certain nanoparticles are more efficient in incorporating carbon atoms and achieving a faster carbon nanotube growth - Researchers of the Center for Multidimensional Carbon Materials, within the Institute of Basic Science (IBS, South Korea), have presented a theoretical solution to a long-lasting mystery of carbon nanotubes (CNTs) growth. Published in Physical Review Letter, this study explains why nanoparticles made with an alloy of metals help to synthetize longer CNTs compared with conventional monometallic catalysts. CNTs are tubular nanostructures made of carbon atoms with exciting potential properties that have kept researchers on the lookout for new advances. One of the most common methods to produce CNTs involves catalyst nanoparticles, which have the function of facilitating the addition of carbon atoms from precursor molecules to the walls of the cylinders. It is common knowledge in the field that alloy catalysts, like Ni-Y, Fe-Mo, Cu-Ni, and Co-Mo, outperform other single metal catalysts, but the reason has been unclear. IBS researchers performed a systematic molecular dynamics simulation to explore the role of alloy catalysts in CNT growth. “In a molecular dynamics simulation, the motion of every atom can be clearly seen and, therefore, the variation of the shape and structure of the catalyst particle during the carbon nanotube growth can be recorded precisely. This allows us to go beyond the capacity of the best experimental methods.” explains Feng Ding, a group leader of the Center and corresponding author of the study.
Through the molecular dynamic simulations, the authors have found that the two metals of the alloy are spatially separated at the rim of the tubes: CNTs tend to attract the more active metal atoms to the open end of the cylinders (growth front), where carbon atoms are inserted into the CNT wall during growth, while the less active metal atoms are pushed above. More simulations show that this is a general phenomenon and can be applied to many types of alloy catalysts. IBS researchers have also demonstrated that alloy catalysts win over monometallic nanoparticles because the active metal atoms near the rim of the CNT catch the carbon atoms more easily than the less active ones. This will lead to a larger carbon concentration at the vicinal of CNT growth front and a quick addition of the carbon atoms, which contribute to the fast growth of the CNT. Since the carbon atoms are continuously incorporated to the growing CNTs, the carbon precursors do not accumulate around the alloy nanoparticles. This prevents the formation of a cap made of carbon atoms engulfing the entire nanoparticle. “This theoretical study addresses a long-term puzzle of the role of the alloy catalysts in carbon nanotube growth. It reveals the advantage of using alloy catalysts in carbon nanotube growth, and the contact-induced phase separation of the alloy catalyst can be considered as a general rule to guide catalyst design for controllable carbon nanotube growth,” says Lu Qiu, the first author of the study. Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20