주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
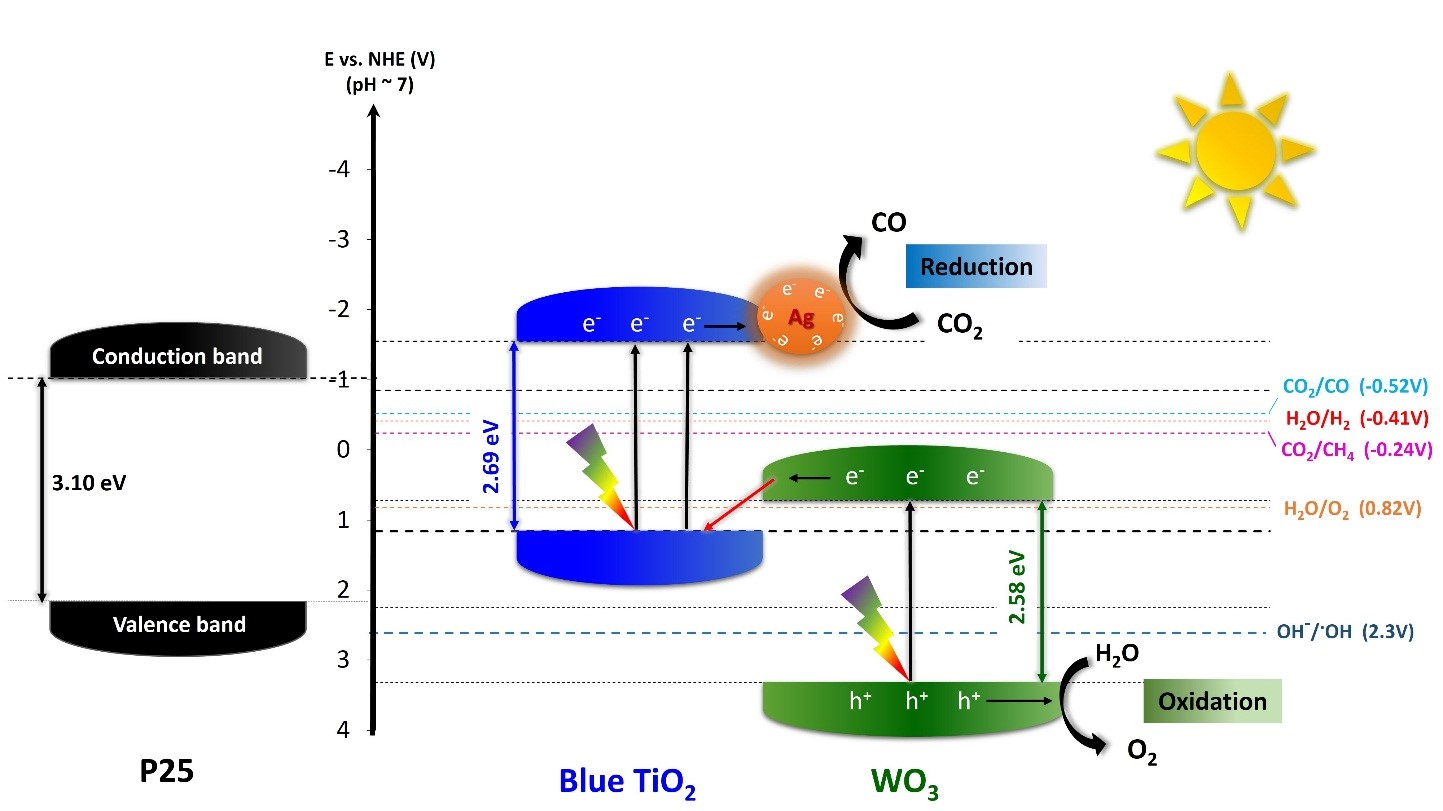
Addressing Global Warming with new Nanoparticles and SunshineArtificial photosynthesis:visible-light-driven photocatalysts convert CO2 into oxygen and Harvesting sunlight, researchers of the Center for Integrated Nanostructure Physics, within the Institute for Basic Science (IBS, South Korea) published in Materials Today a new strategy to transform carbon dioxide (CO2) into oxygen (O2) and pure carbon monoxide (CO) without side-products in water. This artificial photosynthesis method could bring new solutions to environmental pollution and global warming. While, in green plants, photosynthesis fixes CO2 into sugars, the artificial photosynthesis reported in this study can convert CO2 into oxygen and pure CO as output. The latter can then be employed for a broad range of applications in electronics, semiconductor, pharmaceutical, and chemical industries. The key is to find the right high-performance photocatalyst to help the photosynthesis take place by absorbing light, convert CO2, and ensuring an efficient flow of electrons, which is essential for the entire system. Titanium oxide (TiO2) is a well-known photocatalyst. It has already attracted significant attention in the fields of solar energy conversion and environmental protection due to its high reactivity, low toxicity, chemical stability, and low cost. While conventional TiO2 can absorb only UV light, the IBS research team reported previously two different types of blue-colored TiO2 (or “blue titania”) nanoparticles that could absorb visible light thanks to a reduced bandgap of about 2.7 eV. They were made of ordered anatase/disordered rutile (Ao/Rd) TiO2 (called, HYL’s blue TiO2-I) (Energy & Environmental Science, 2016), and disordered anatase/ordered rutile (Ad/Ro) TiO2 (called, HYL’s blue TiO2-II) (ACS Applied Materials & Interfaces, 2019), where anatase and rutile refer to two crystalline forms of TiO2 and the introduction of irregularities (disorder) in the crystal enhances the absorption of visible and infra-red light.
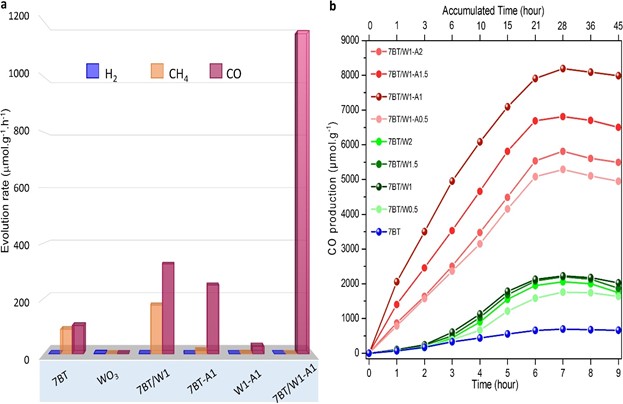
For the efficient artificial photosynthesis for the conversion of CO2 into oxygen and pure CO, IBS researchers aimed to improve the performance of these nanoparticles by combining blue (Ao/Rd) TiO2 with other semiconductors and metals that can enhance water oxidation to oxygen, in parallel to CO2 reduction into CO only. The research team obtained the best results with hybrid nanoparticles made of blue titania, tungsten trioxide (WO3), and 1% silver (TiO2/WO3–Ag). WO3 was chosen because of the low valence band position with its narrow bandgap of 2.6 eV, high stability, and low cost. Silver was added because it enhances visible light absorption, by creating a collective oscillation of free electrons excited by light, and also gives high CO selectivity. The hybrid nanoparticles showed about 200 times higher performance than nanoparticles made of TiO2 alone and TiO2/WO3 without silver. Starting from water and CO2, this novel hybrid catalyst produced O2 and pure CO, without any side products, such as hydrogen gas (H2) and metane (CH4). The apparent quantum yield that is the ratio of several reacted electrons to the number of incident photons was 34.8 %, and the rate of reacted electrons 2333.44 μmol g−1h−1. The same measurement was lower for nanoparticles without silver (2053.2 μmol g−1h−1), and for nanoparticles with only blue TiO2 (912.4 μmol g−1h−1).
Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20