주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
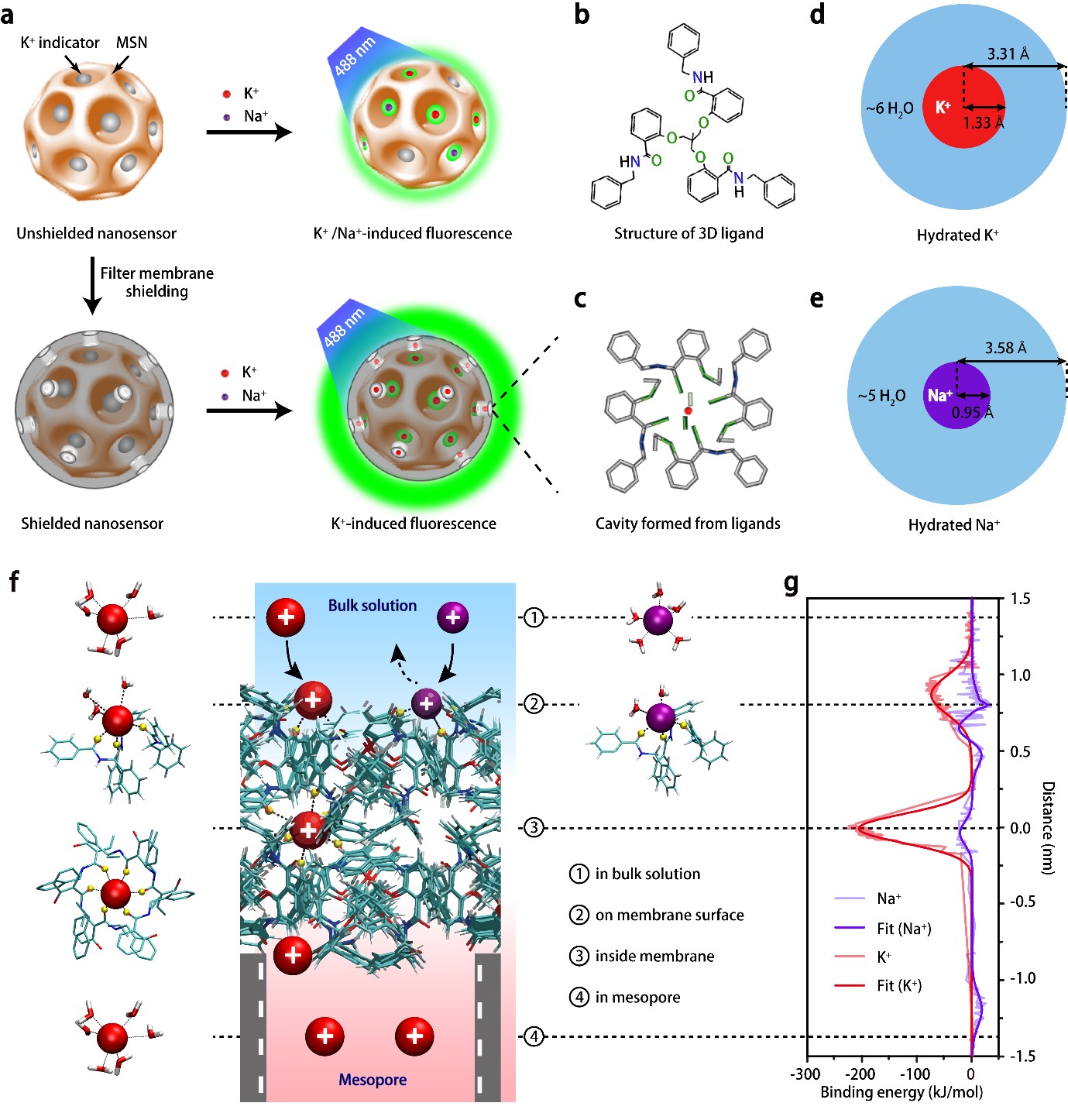
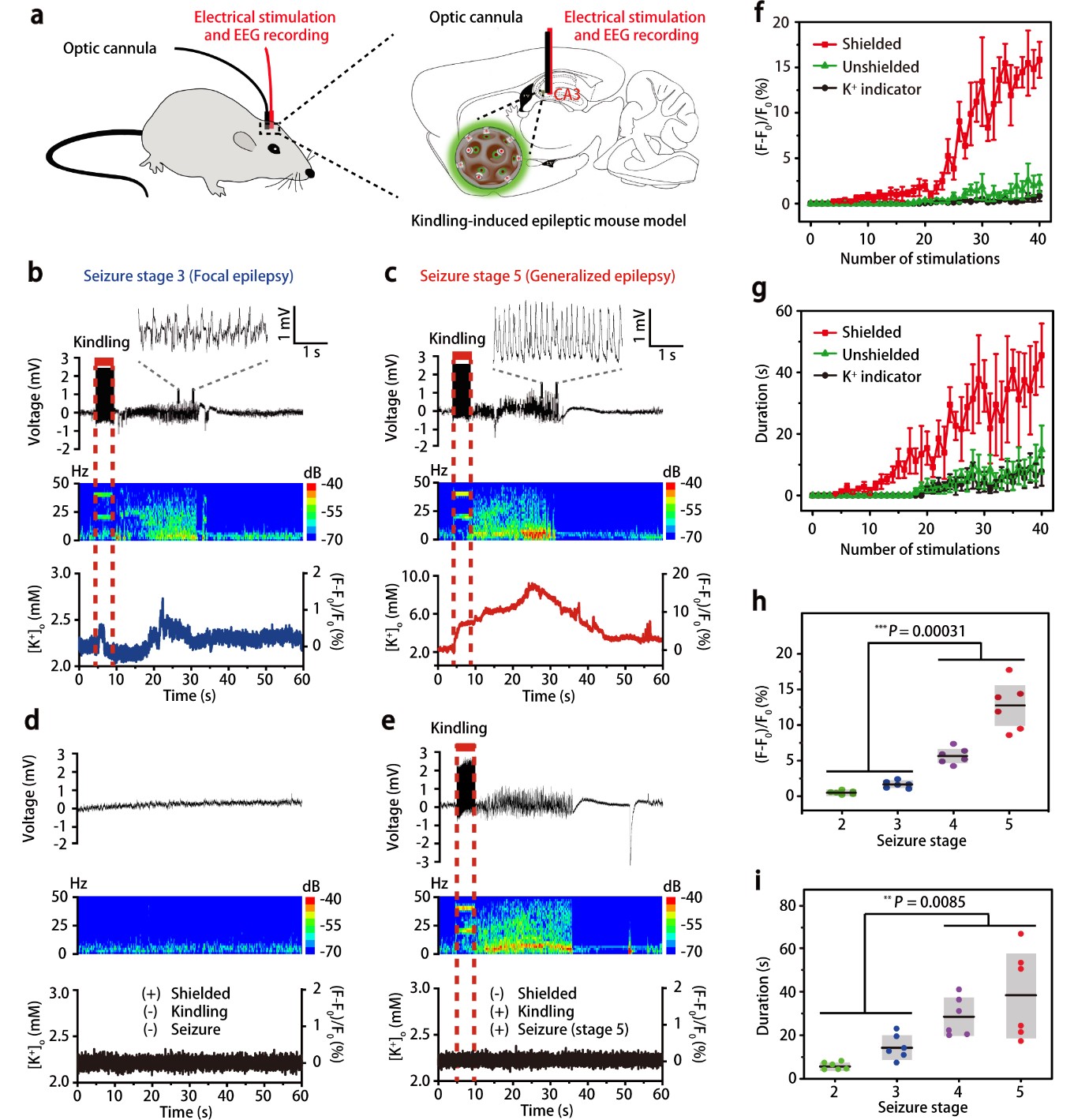
Sensitive and specific potassium nanosensors to detect epileptic seizures- A filter membrane on nanosensors enables the accurate sensing of potassium levels in the brain and helps to elucidate when and how epileptic seizures happen - Researchers at the Center for Nanoparticle Research, within the Institute for Basic Science (IBS, South Korea) in collaboration with collaborators at Zhejiang University, China, have reported a highly sensitive and specific nanosensor that can monitor dynamic changes of potassium ion in mice undergoing epileptic seizures, indicating their intensity and origin in the brain. Epilepsy is a central nervous system disorder accompanied by abnormal brain activity, causing seizures or periods of unusual behavior, sensations, and sometimes loss of awareness. If epileptic seizures last for 30 minutes or longer, they can cause permanent brain damage or even death. The need of technologies to evaluate the degree of abnormal electrical activity associated with epilepsy is well known. One of the main investigation targets is potassium (K+) ion. This ion affects the difference in electric potential between the interior and exterior membrane of the neurons, and impacts the neuronal intrinsic excitability and synaptic transmission. Despite the significant efforts to improve the selectivity of K+ sensors, they are still far from satisfactory because currently available optical reporters are not capable of detecting small changes in potassium ion, in particular, in freely moving animals. Furthermore, they are susceptible to interference from sodium ions because Na+ influx is shortly followed by K+ efflux when impulses pass along the membrane of nerve cell. In this study published in Nature Nanotechnology, the researchers report a highly sensitive and selective K+ nanosensor that can monitor the changes of K+ in the different parts of the brains of freely moving mice. The new nanosensor is created with porous silica nanoparticles shielded by an ultrathin potassium-permeable membrane that is very similar to the potassium channel in brain cells. The size of the pores allows only K+ to diffuse in and out, reaching a detection limit as low as 1.3 micromolar. This allows the specific readout of sub-millimolar variations of extracellular K+ and the spatial mapping of this ion in the brain.
This study successfully demonstrated that K+-permeable membrane filter on the nanosensor is effective at filtering out other cations and capturing K+ ions exclusively. Such a nanosensor construction strategy would contribute not only to scientific discoveries and breakthroughs in neuroscience research, but also to the development of other selective ion sensors. Using these nanosensors in the hippocampal CA3 region, the team was able to report the degree of epileptic seizures in living mice and compare it with the recordings of neural activity done with electroencephalography (EEG).
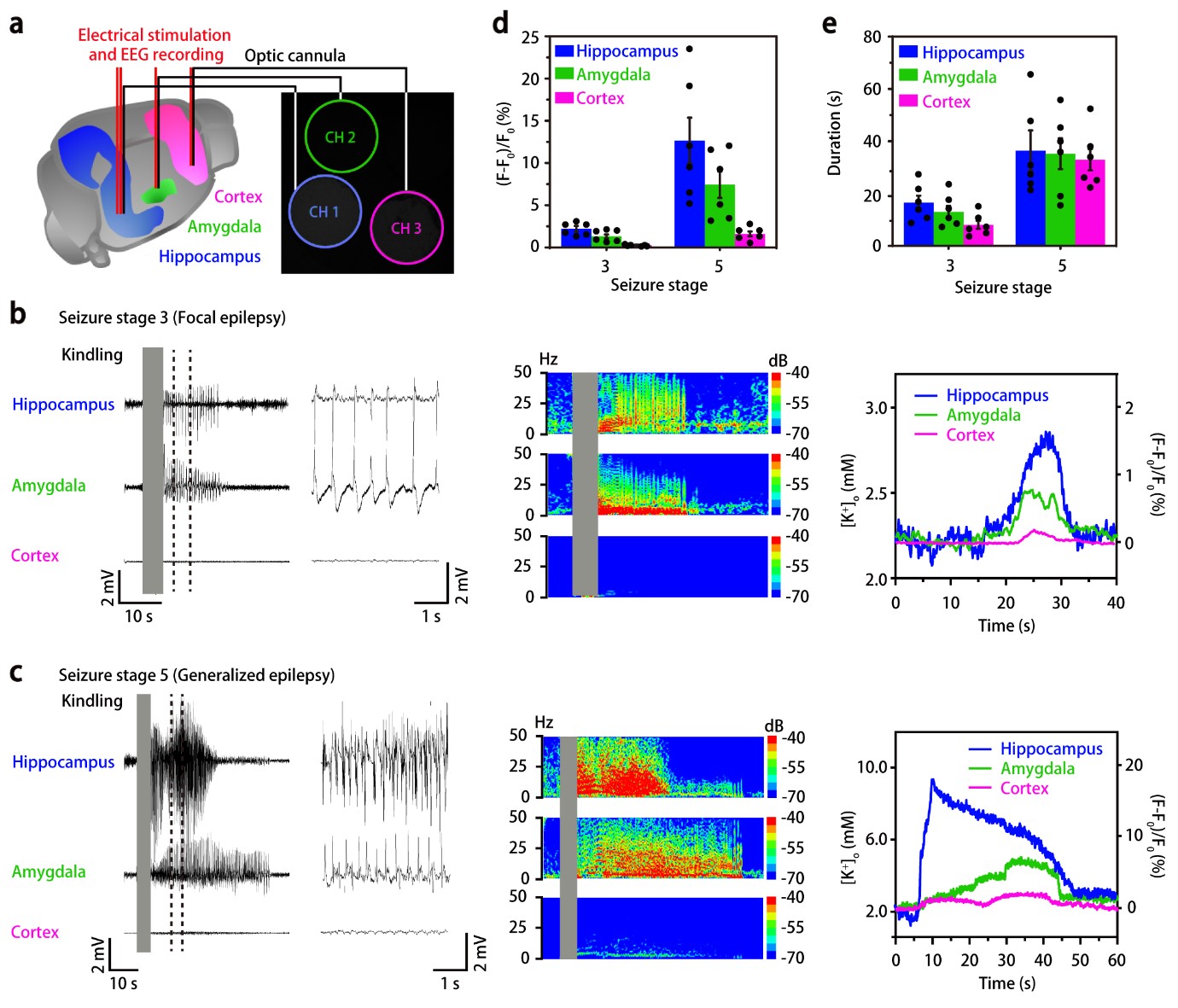
To further check whether the nanosensors are capable of measuring K+ in multiple sub-regions of the brain in freely moving mice, the researchers injected the nanosensors into three different locations of the mouse brains: hippocampus, amygdala, and cortex. After the electrical stimulation at the hippocampus, the EEG and optical responses of the nanosensors at the injected locations were simultaneously recorded. Interestingly, the external K+ concentration increases from hippocampus to amygdala and cortex over time in focal seizures, while it increases almost simultaneously in the three brain regions in generalized seizures. These results are in good agreement with the widely accepted view that electrical stimulation in the hippocampus first involves the adjacent brain area and then propagates throughout the entire brain. HYEON Taeghwan, director of the IBS Center for Nanoparticle Research (Distinguished Professor at Seoul National University) and leading author of the study notes, “Further development of these nanosensors could facilitate diagnosis and therapy, decreasing the need for surgery. Ideally, these nanosensors could also carry antiepileptic drugs to be released in the right points of the brain where seizures originated.”
Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20