주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
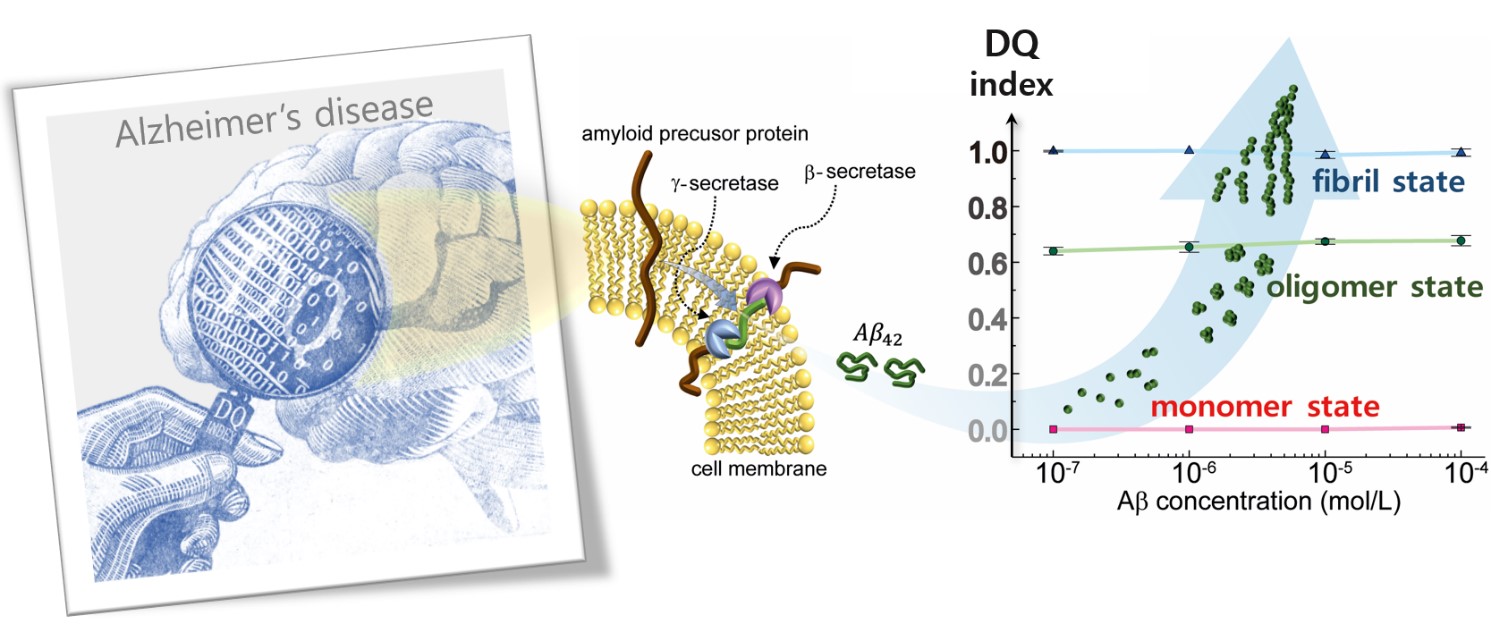
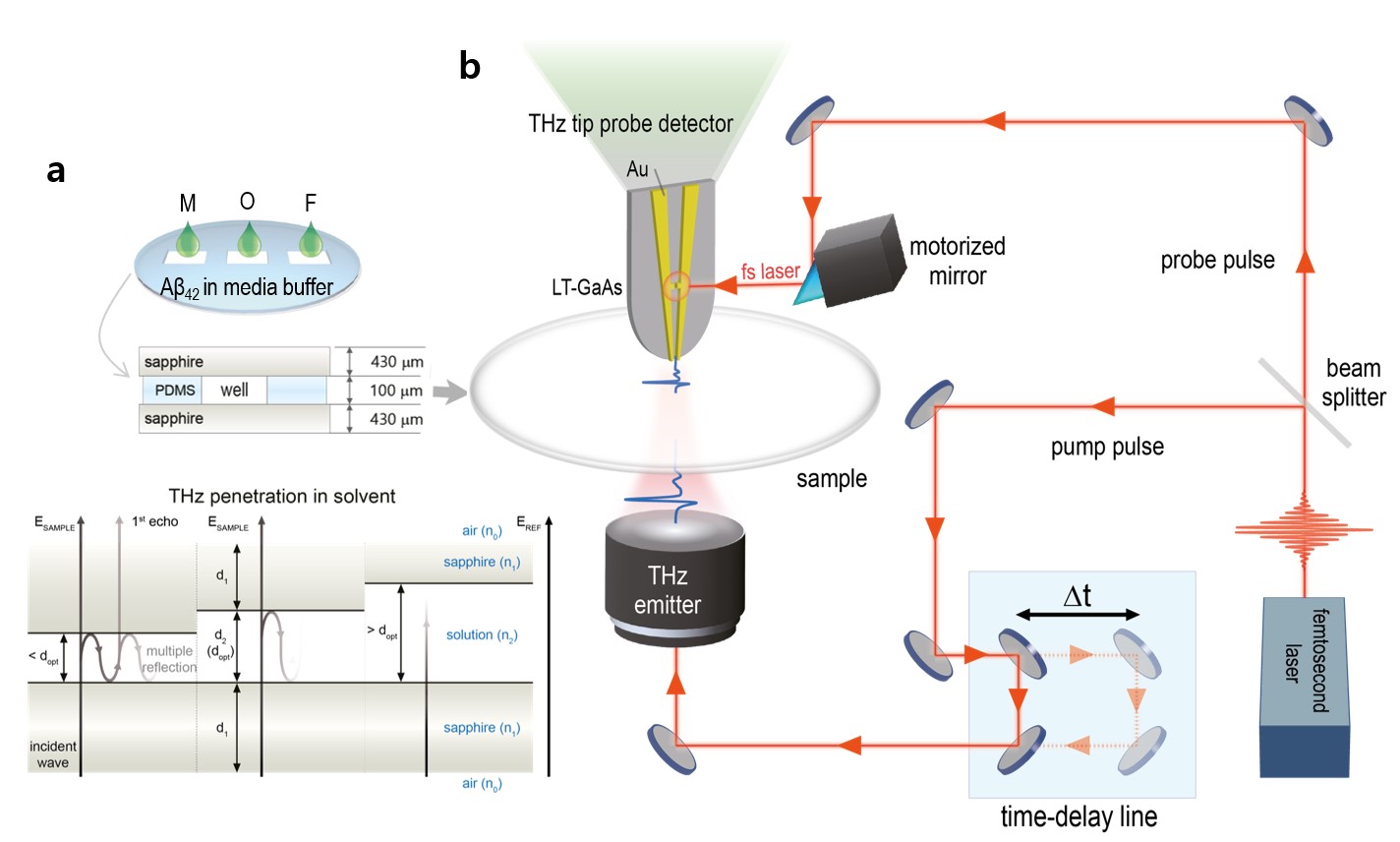
New method to monitor Alzheimer’s proteins- Terahertz waves detect the fibrillization state of amyloid beta proteins in solution - Physicists at the Center for Integrated Nanostructure Physics (CINAP), within the Institute for Basic Science (IBS, South Korea), have reported a new method to identify the aggregation state of amyloid beta (Aβ) proteins in solution. Published in the American Chemical Society Nano (ACS Nano), this finding could represent a step forward in the early diagnosis of Alzheimer’s disease. The gradual accumulation of Aβ in the brain leads to incurable dementia. The disease progression is strongly correlated with the form of the Aβ proteins: 4-nm-size monomers evolve to oligomer of several hundred nanometers and reaches the fibrillar state forming plaques of up to a few tens of micrometers in size. The researchers clearly discerned the different Aβ stages using THz near-field conductance measurements. This technique measures the energy absorbed by molecules at an energy band of around 1-10 meV (or 0.2-2.4 THz), and it is considered an effective technique for investigating the transformation of biological macromolecules without generating heat. The scientists measured how Aβ proteins in the solution are disturbed by incident THz radiation and noticed that the results were correlated with the form of the Aβ proteins: monomer, oligomer and fibril. Then, they derived the optical conductance, which decreases with the evolving fibrillization states and increases with the elevating molar concentrations. Since the progressive stages of the disease can be differentiated simply with this technique, the team derived a dementia quotient (DQ) as a localization parameter from the optical conductance, using the so-called Drude−Smith model. A DQ value of around one indicates that Aβ is in a fibril state, around 0.64 an oligomeric state, and nearly zero is at a monomeric state.
“We believe that our result gives us a significant paradigm shift in the Alzheimer’s disease research field, since the dementia quotient is clearly identified from the label-free conductance measurement of different Aβ protein structural states,” says Chaejeong Heo, one of the leading authors of this study. CINAP Director, Young Hee Lee adds “This index can be useful for early detection of toxic Aβ protein aggregation and fibrillization observed in Alzheimer’s disease.”
Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20