주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
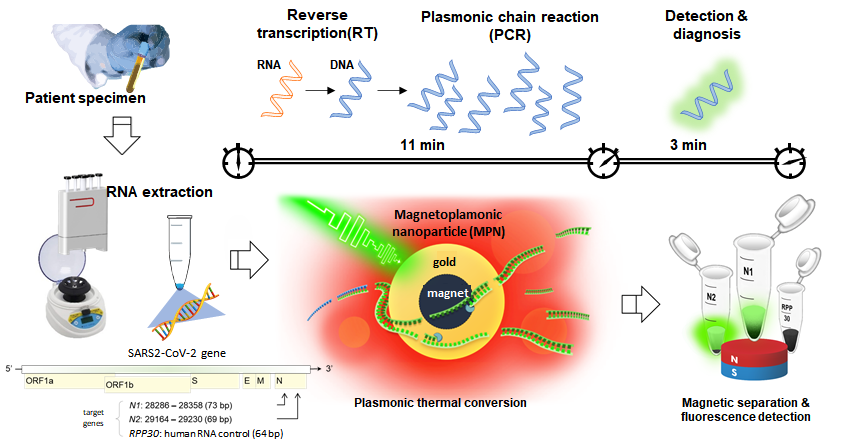
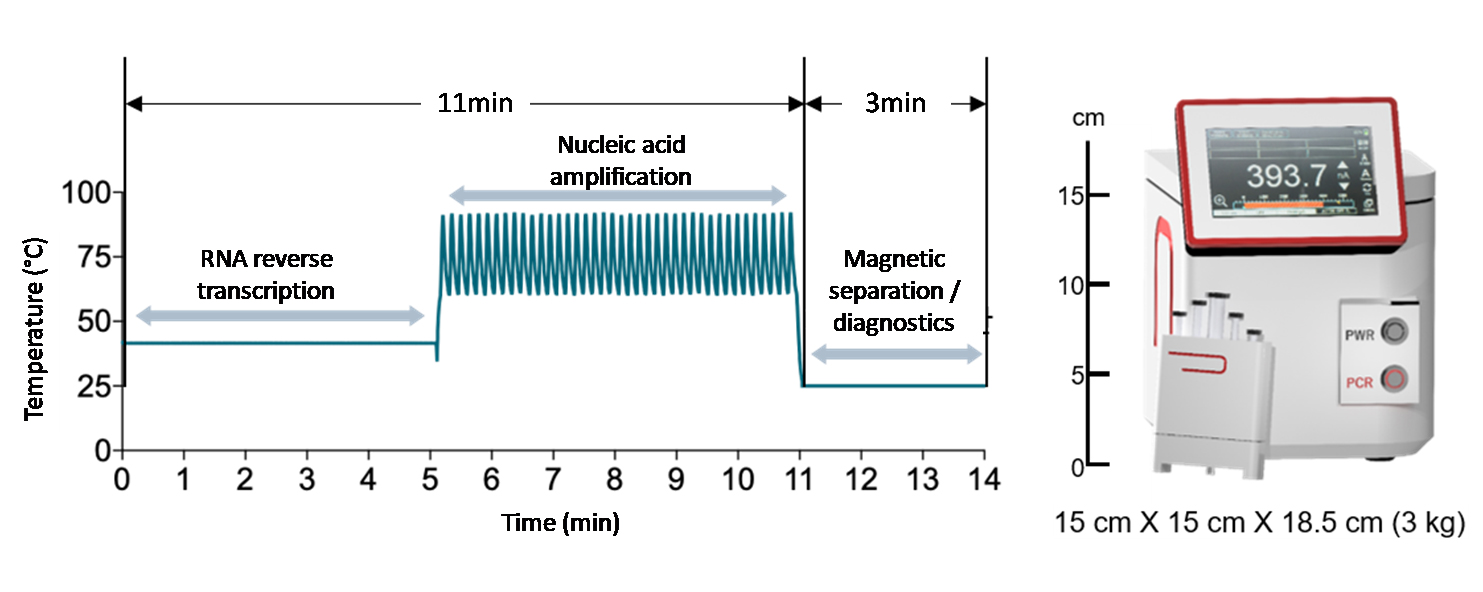
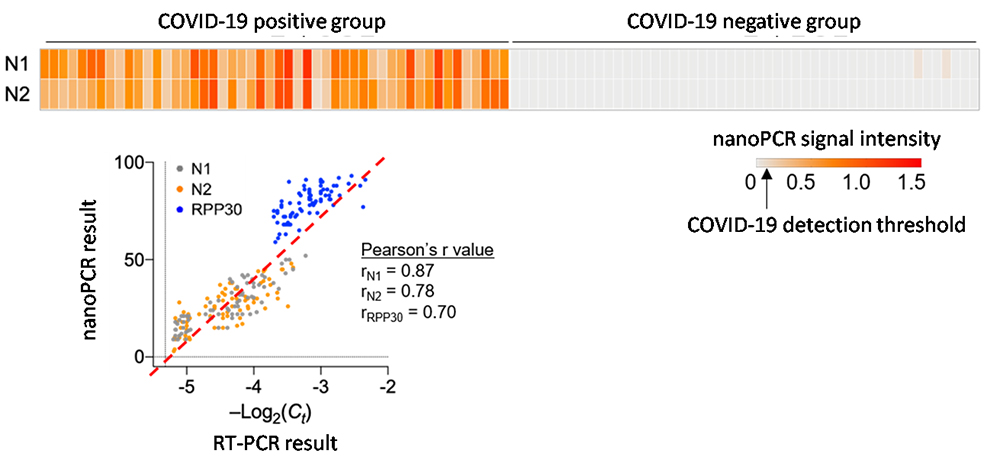
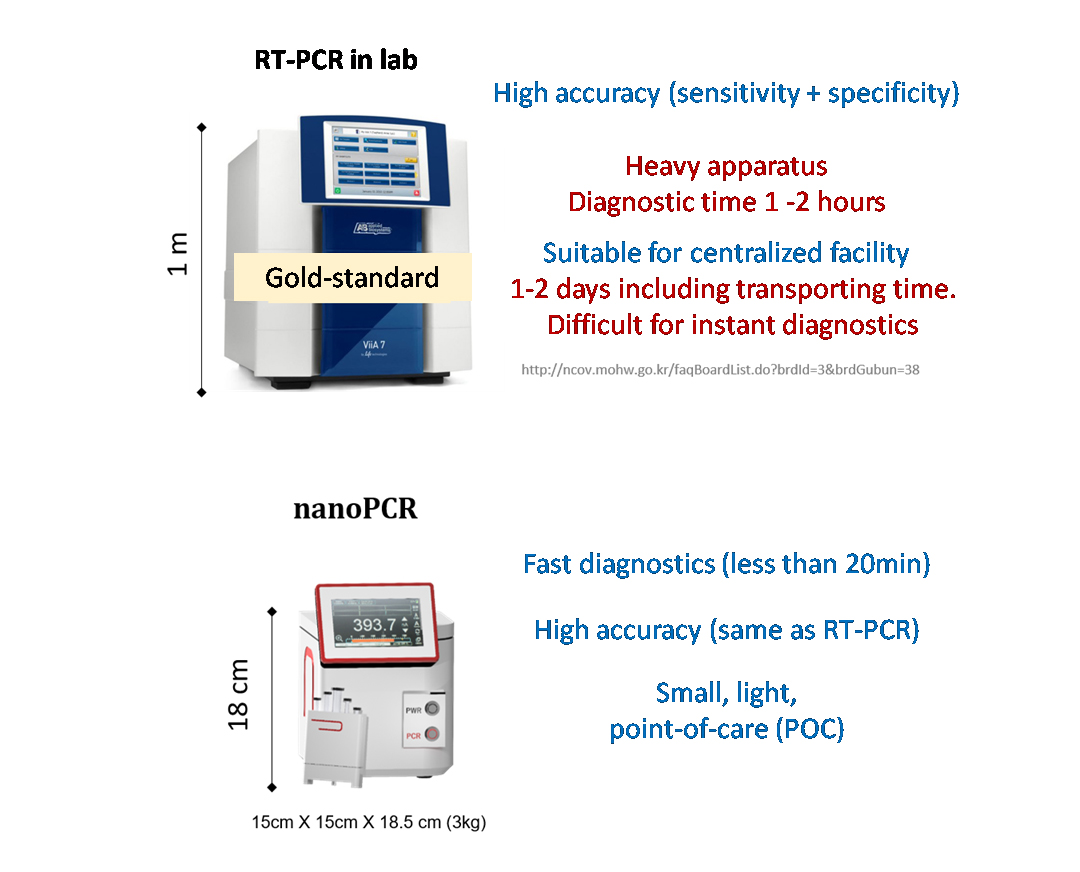
Development of high-speed nanoPCR technology for point-of-care diagnosis of COVID-19The new technology can accurately diagnose the infection in less than 20 minutes, with zero false positive and false negative A “nanoPCR” technology was developed for the point-of-care (POC) diagnosis of coronavirus disease-19 (COVID-19). This new technology can diagnose the infection within ~20 minutes while retaining the accuracy of conventional reverse transcription polymerase chain reaction (RT-PCR) technology. A team of researchers led by Professor CHEON Jinwoo, the director of the Center for Nanomedicine (CNM) within the Institute for Basic Science (IBS) in Seoul, South Korea, in collaboration with Professor LEE Jae-Hyun from Yonsei University and Professor LEE Hakho from Massachusetts General Hospital developed a novel nanoPCR technology that can be used for the decentralized, POC diagnosis of COVID-19. The technique uses the same underlying principle as the standard diagnosis method of RT-PCR to detect viral RNA, but it also features a vast improvement in speed using hybrid nanomaterials and a miniaturized form factor which allows portability. The gold-standard test method for COVID-19 currently used is RT-PCR: a test that amplifies DNA after changing RNA genes into complementary DNAs for detection. RT-PCR has high accuracy, but it takes one to two hours to detect viruses at the centralized facility equipped with bulky instrumentations. The logistics process of cold chain transportation from the sampling sites to the testing facility makes the conventional RT-PCR diagnosis even slower, taking 1 – 2 days to get the results back to the patients. To overcome the limitations of existing diagnostic methods, the research team utilized a magneto-plasmonic nanoparticle (MPN) that is comprised of magnetic material in its core and a gold shell that exhibits plasmonic effects. By applying MPNs to PCR, they developed 'nanoPCR' which greatly improves the speed of RT-PCR while retaining highly accurate detection. Plasmonic properties of MPN refer to its ability to convert light energy into thermal energy, and by using this it was possible to shorten the thermocycling step of RT-PCR from 1 – 2 hours to within 11 minutes. In addition, the strong magnetic property of MPN allows an external magnetic field to clear MPNs from the PCR solution to allow for fluorescent detection of the amplified genes. The nanoPCR is capable of detecting even a small amount of genes (~3.2 copies/µl) accurately while simultaneously amplifying and detecting genetic material with high sensitivity and specificity. The researchers tested nanoPCR under clinical settings through the patient specimen tests conducted with Professor CHOI Hyun-Jung's team at Chonnam National University Hospital. During the test, 150 subjects with or without COVID-19 infection were accurately diagnosed using this technology (75 positives, 75 negative samples; zero false-negatives and false-positives). The level of sensitivity and specificity was found to be equivalent to that of the conventional RT-PCR (~99%). In addition to high reliability, the whole diagnostic process was considerably fast, as on average it took about 17 minutes for the diagnosis of one specimen. In addition, the researchers showed the possibility of improving the analytical throughput by applying a Ferris wheel system to load multiple samples at once, which would allow for simultaneous testing of many samples from multiple patients. Importantly, the nanoPCR equipment is very compact in size (15 × 15 × 18cm) and weight (3 kg), which allows it to be portable. All of this would pave the way for rapid, decentralized testing of patients for the POC diagnosis. Director Cheon said, "Through the improvement and miniaturization of the PCR technology, we have shown that it is possible to perform PCR based POC diagnosis in the field quickly." The research is currently at a proof-of-concept stage and further developments are needed until it can be used in the field. The results of this study were published in Nature Biomedical Engineering on December 3rd, 2020.
Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20