주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
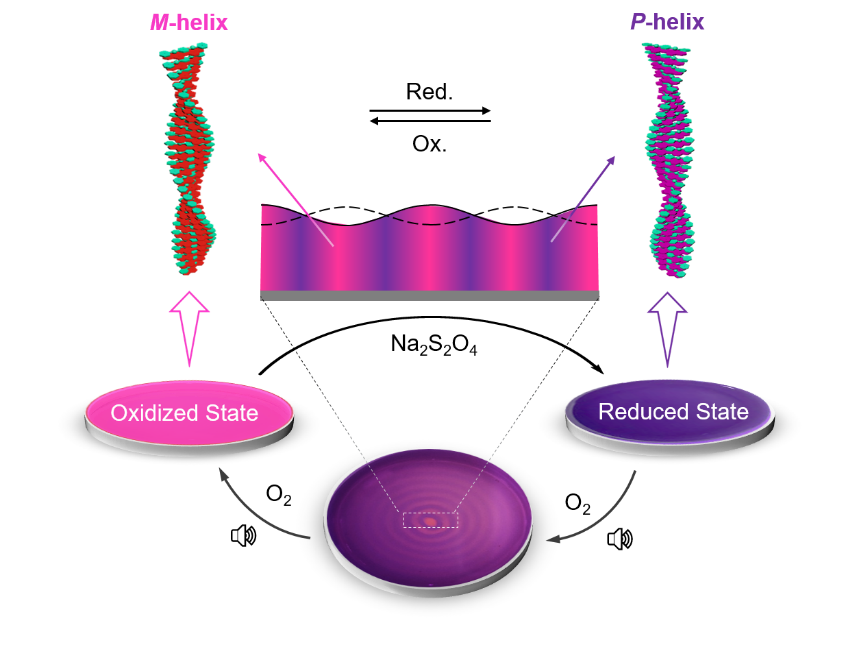
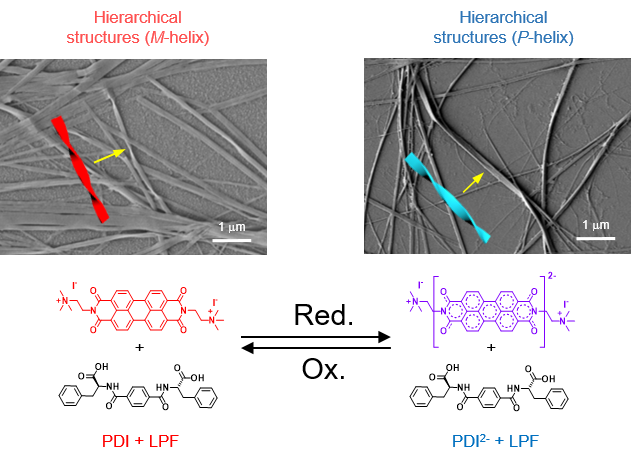
‘Sound’ly Segregated Supramolecular Helices- Scientists achieve spatiotemporal segregation of redox-responsive chiral supramolecular polymers in solution using audible sound - In 1848, Louis Pasteur successfully segregated two types of crystals of double sodium-ammonium salt of tartaric acid, which were mirror images of each other. Back then, this was done manually through a painstaking process of observing the crystals under a microscope and utilizing a simple pair of tweezers. Following this seminal discovery, the field of stereochemistry has made great progress. Today, advances in technology now allow chemists to explore programmable control over the helicity of chiral supramolecular polymers with molecular-level manipulation, stimuli-responsive chirality switching, etc. Unlike sub-millimeter scale stable crystals of sodium-ammonium tartrate, chiral supramolecular polymers are soft materials with dynamic structures and their size ranges from the nanometer scale to a few microns. Moreover, chiral supramolecular polymers are not uniformly present in solution due to uncontrolled diffusion. Their distribution can be even more random and complex, especially when dealing with a mixture of supramolecular species in multicomponent self-assemblies. Manual segregation of such chiral supramolecular polymers is therefore difficult to achieve and practically impossible. Now, a team led by Director KIM Kimoon at the Center for Self-assembly and Complexity within the Institute for Basic Science in Pohang, South Korea has successfully demonstrated that audible sound can be utilized to spatiotemporally segregate supramolecular polymers, which differ in their chirality, within separate domains within the same solution. Dr. Shovan Kumar SEN, the first author of this study is very much excited about this development. He said, “Redox-responsive chiral switches are well-studied in the literature, but not all systems behave as we anticipate in the presence of audible sound. Designing the most appropriate system to demonstrate our idea, which was initiated with our previous work published in Nature Chemistry (in 2020), was one of the most important junctures of this study.” To achieve these results, the researchers utilized out-of-equilibrium redox-responsive supramolecular systems, which form supramolecular polymers of opposite chirality in their oxidized and reduced states. Further, utilizing audible sound (40 Hz), the researchers were able to segregate supramolecular polymers of opposite helicity through spatiotemporal pattern formation. Audible sound induces surface vibrations and advection currents within the bulk solution placed in a Petri dish. This results in a faster dissolution of atmospheric oxygen at the concentric ring-shaped domains, which are located at the antinodal (maximum vibrating) regions of the surface waves, resulting in the accumulation of the oxidized polymer strands in these regions. The aforementioned rings are separated by another set of concentric domains, which are located at the nodal (minimum vibrating) regions of the surface waves and contain the oppositely chiral reduced form of the chiral supramolecular polymer (Figure 1). The researchers designed a redox-responsive achiral system based on a perylene diimide molecule functionalized with quaternary amine groups (PDI). They then explored the co-assembly of PDI with a negatively charged (at alkaline pH) phenylalanine-derived gelator (LPF) making use of electrostatic interactions. The co-assembled PDI-LPF aggregates led to the formation of left-handed helical supramolecular aggregates (M-helix). Interestingly, when sodium dithionite (SDT) was added as a reducing agent to the PDI-LPF co-assembly, the oppositely helical supramolecular aggregates (P-helix) were formed due to the formation of PDI2-LPF aggregates. The oppositely chiral hierarchical structures (helical nanofibers) were observed from electron microscopy studies, which further confirmed the helicity inversion (Figure 2). Dr. Rahul Dev MUKHOPADHYAY, who led this study believes there is more in store with this sound-based approach. He opined, “As of now, we can only achieve transiently controlled segregation of two oppositely chiral supramolecular polymers within a solution. It will be very interesting and challenging to achieve permanent segregation of such oppositely helical dynamic supramolecular polymers.” The team also explored the chiral co-assembly between PDI and adenosine triphosphate (ATP). Interestingly, the chiral co-assembly disintegrated on reduction to form achiral PDI2-, releasing ATP back into the solution. When a reduced solution of a PDI-ATP co-assembly was exposed to atmospheric oxygen in the presence of audible sound at a specific frequency (40 Hz), the solution gradually reorganized into a spatiotemporal pattern consisting of segregated domains corresponding to the achiral PDI2- aggregates and chiral PDI-ATP aggregates. Prof. KIM Kimoon, who was responsible for the overall supervision of the study, strongly believes that the present strategy provides a new tool for researchers in the research field of systems chemistry to control supramolecular systems. Currently, the approach helps us segregate only two types of polymers within the same solution. In the future, Prof. Kim hopes we will have a chance to control multiple aggregates using audible sound. He quickly added, “Always remember what Pasteur said, chance favors the prepared mind.”
Notes for editors
- Reference
- Media Contact
- About the Institute for Basic Science (IBS)
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20