주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
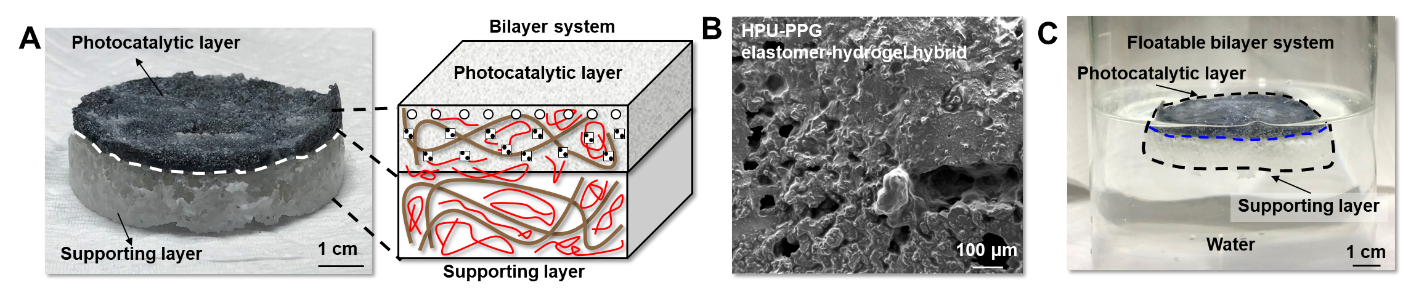
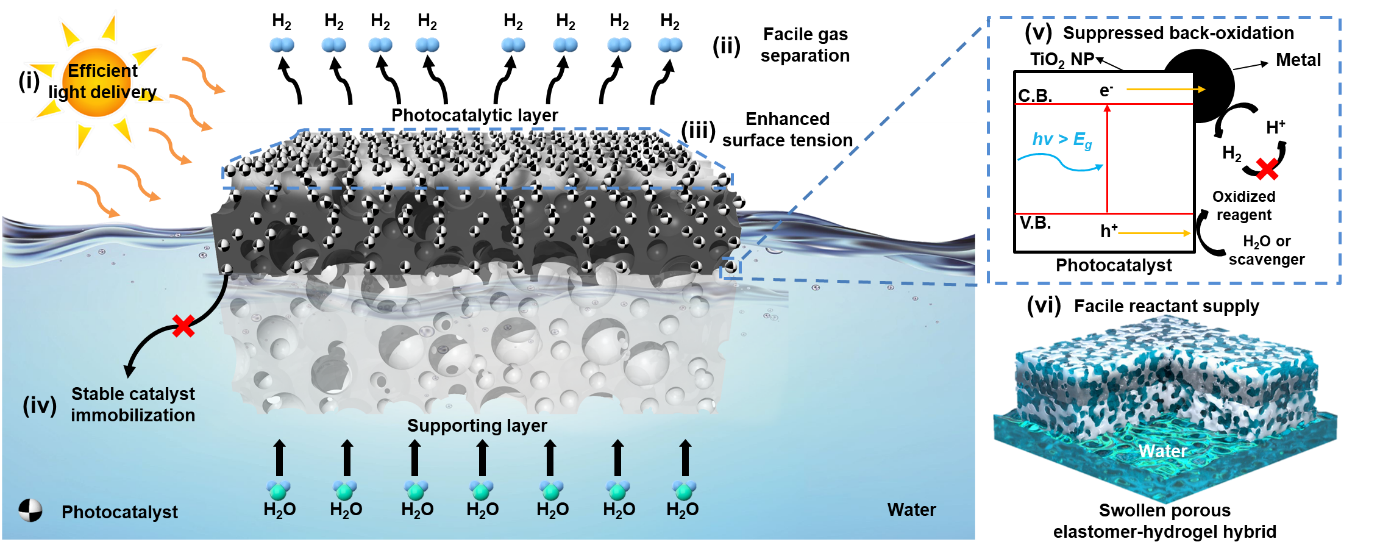
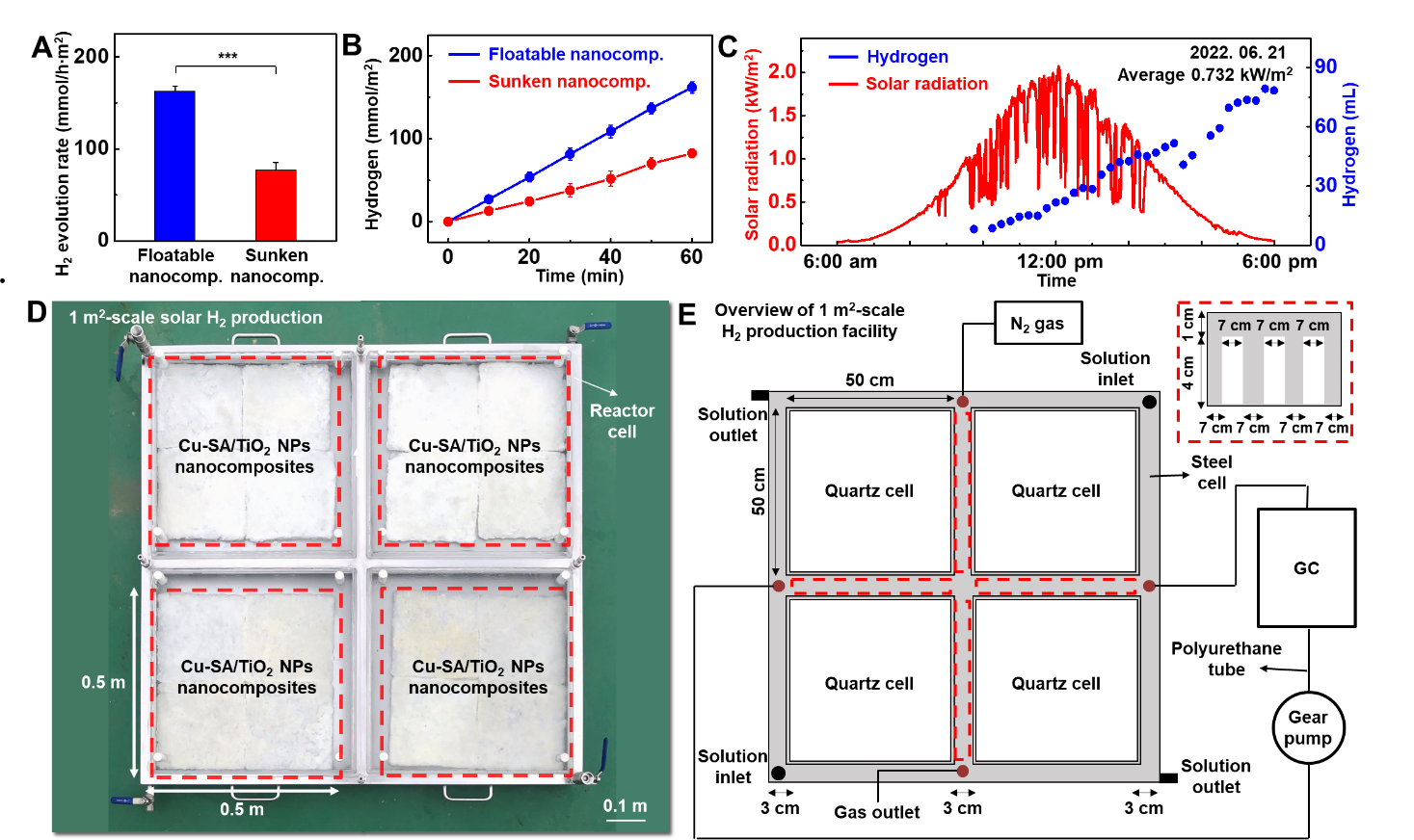
Newly developed hydrogel nanocomposite for the mass production of hydrogen- A new type of floatable photocatalytic platform composed of hydrogel nanocomposites efficiently proceeds hydrogen evolution reaction, even by using plastic wastes - A research team led by Prof. HYEON Taeghwan at the Center for Nanoparticle Research within the Institute for Basic Science (IBS) in Seoul, South Korea has developed a new photocatalytic platform for the mass production of hydrogen. The group’s study on the photocatalytic platform led to the development of a floatable photocatalytic matrix, which allows efficient hydrogen evolution reaction with clear advantages over conventional hydrogen production platforms such as film or panel types. The importance of alternative energy has recently increased due to global challenges such as environmental pollution and climate change. Among several candidates for alternative energy sources, hydrogen energy harvested by photocatalysis is particularly highlighted for its sustainable green energy production. Accordingly, much research and development have been made to enhance the intrinsic reaction efficiency of photocatalysts. However, research on the form factor of photocatalytic systems, which is critical for their practical application and commercialization, has not yet been actively explored. Usually, current systems fix catalyst powder or nanoparticles onto different surfaces, such as particulate sheet-type, film-type, and flat panel-type platforms, which are submerged under water. They also face practical issues such as the leaching of catalysts, poor mass transfer, and reverse reactions. They also require additional devices to separate and collect the generated hydrogen from water, which adds to the complexity of the device and increases the costs. The team at the Center for Nanoparticle Research within the IBS, led by Prof. Hyeon, designed a new type of photocatalytic platform that floats on the water for efficient hydrogen production. This new platform has a bilayer structure, which consists of an upper photocatalytic layer and a lower supporting layer (Figure 1A). Both layers are composed of a porous structural polymer that endows high surface tension to the platform (Figure 1B). In addition, the platform is fabricated in the form of cryo aerogel, a solid substance filled with gas inside, exhibiting low density. As a result, this elastomer-hydrogel embedded with photocatalysts can float on water (Figure 1C). This platform exhibits clear advantages in the photocatalytic hydrogen evolution reaction: first, light attenuation by water is prevented, resulting in efficient solar energy conversion. Second, the product, hydrogen gas, can be easily diffused into the air, avoiding reverse oxidation reactions and preserving high reaction yield. Third, the water can be easily supplied to the catalysts located inside the elastomer-hydrogel matrix due to its porosity. Last, catalysts are stably immobilized inside the matrix for long-term operation without leaching issues (Figure 2). The researchers experimentally proved the superior hydrogen evolution performance of the floatable platform, compared to that of the conventional submerged platform (Figure 3A, B). Furthermore, the scalability of the platform, which is essential for potential industrialization, was also demonstrated under natural sunlight. It was confirmed that about 80 mL of hydrogen can be produced by the floatable photocatalytic platform using copper single atom and titania catalysts with an area of 1 m2 (Figure 3C-E). Even after 2 weeks of operation in seawater containing various microorganisms and floating matter, the hydrogen evolution performance of the platform was not compromised. Prof. Kim states, “The proposed platform can even produce hydrogen from solutions that dissolve household waste, such as polyethylene terephthalate bottles. Consequently, the platform can be a solution for recycling wastes, which contributes to an environment-friendly society.” Notably, this study presents a generalized platform for efficient photocatalysis that is not just limited to hydrogen production. It is possible to replace the catalytic component for various desired uses, without changing the floatable aerogel material properties of the overall platform. This guarantees the wide applicability of the platform to other photocatalytic reactions, such as oxygen evolution reaction, hydrogen peroxide production, and generation of various organic compounds. “This study makes great progress in the field of photocatalysis and showcases the potential of green hydrogen production at sea with world-class performance. The distinctive material features, high performance, and broad applicability in the field of photocatalysis of our platform will undoubtedly open a new chapter in alternative energy,” remarked Prof. Hyeon.
Notes for editors
- Reference
- Media Contact
- About the Institute for Basic Science (IBS)
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20