주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
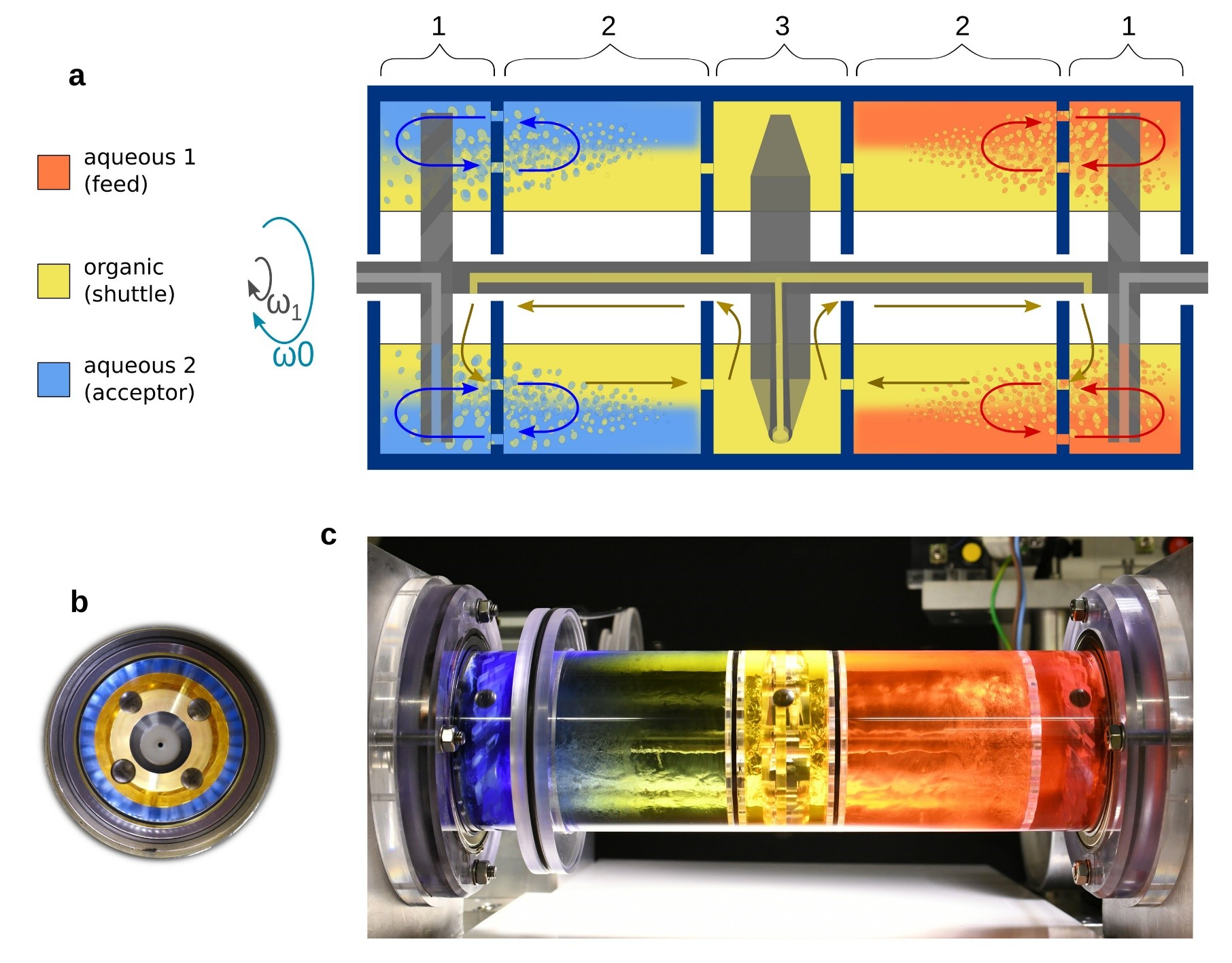
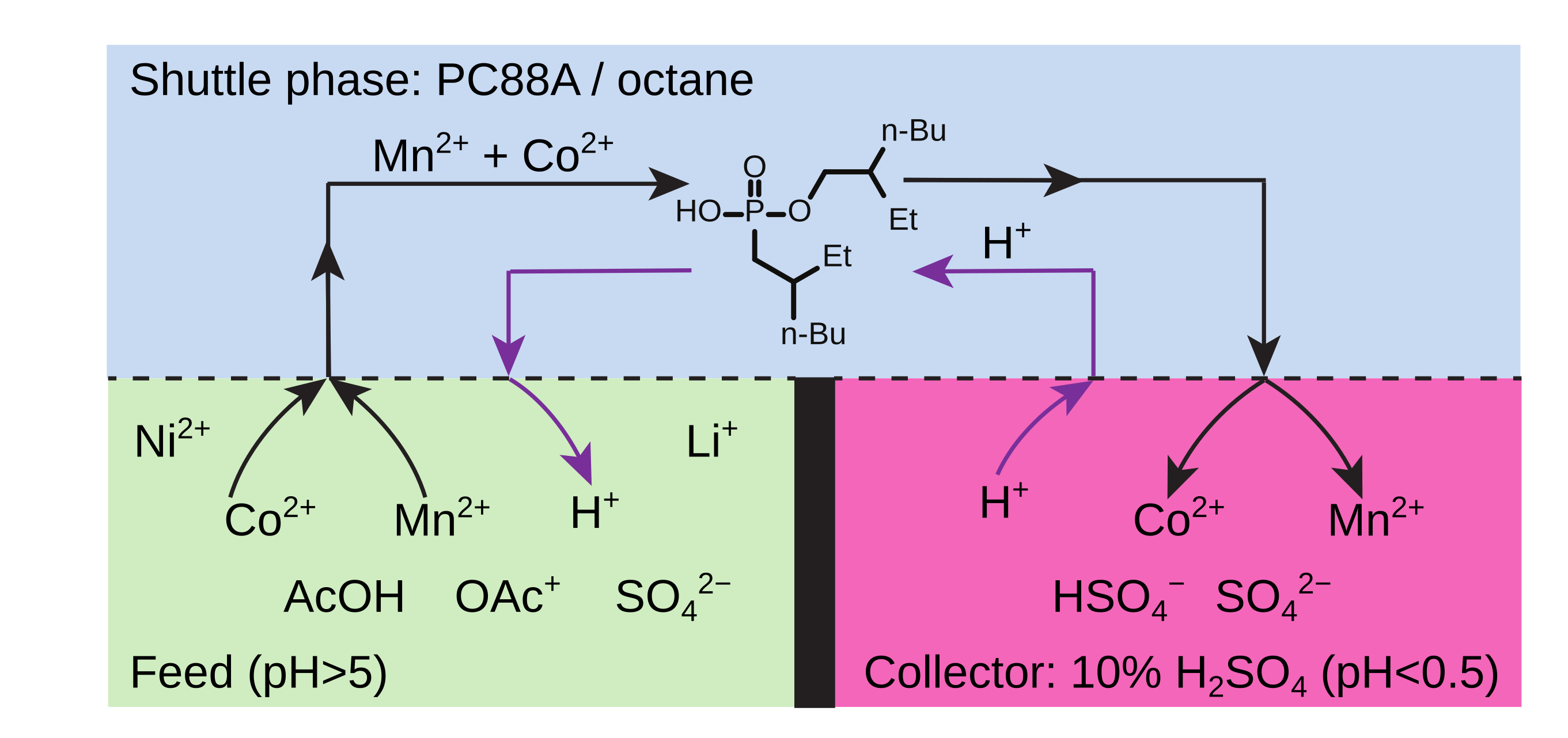
Recycling of Valuable Metals from Spent Lithium Ion Batteries Using Spinning ReactorsIn a world that is slowly distancing itself from carbon-based energy, there has been a meteoric rise in the use of lithium-ion batteries as a next-generation energy storage solution. However, this has resulted in another problem - an increase in the amount of lithium battery waste. Lithium-ion batteries degrade slowly over their lifetime, losing anywhere from 12% to 24% of their total capacity over 500 charging and discharging cycles. The electrolyte and other materials inside the battery can also degrade, causing a decrease in capacity over time. The disposal of lithium batteries in landfills or incineration can pose environmental and safety concerns due to the potential for toxic elements to leach into the soil and water. Recycling lithium batteries requires extensive use of hydrometallurgy, a branch of metallurgy involving aqueous solutions. Briefly, the used battery is dismantled and valuable metals are extracted using solvent, followed by stripping where extracted metal is recovered and the solvent is recycled. The current lithium battery recycling requires multiple steps of extraction-stripping processes, each requiring separate reactors and different parameters. This significantly drives up the complexity and cost of recycling, hence the recycling rate of lithium batteries is very low. There have been numerous attempts to devise a single-step, one-pot solution by partitioning the reactor using membranes. However, these ideas have failed in larger reactors, mostly due to membrane failures, especially under strong stirring. To address this issue, an interdisciplinary research group led by Professor Bartosz A. GRZYBOWSKI at the Center of Soft and Living Matter within the Institute for Basic Science (IBS), South Korea, reported a novel method for recycling valuable metals such as lithium, nickel, and cobalt, from spent lithium-ion batteries. Gryzbowski’s group has been famous in the field for their spinning ‘concentric liquid reactors’, which were proven to be effective in carrying out multi-step reactions in a single chamber. This time, the group successfully applied this concept to simplify the extraction-stripping process for lithium battery recycling. The horizontally rotating reactor, which was designed by co-author Dr. Olgierd CYBULSKI, can process complex metal mixtures in which aqueous feed, organic extractant, and aqueous acceptor phases are all present in the same, rotating vessel. Unlike the one-pot setups that use membranes, this reactor can be vigorously stirred and emulsified without the coalescence of aqueous layers. The arrangement of higher-pH “feed”, organic extractant (“shuttle”), and lower-pH “acceptor” phases is robustly maintained by placing all these liquids in a rotating vessel in a way that they form concentric layers stable enough to allow efficient interfacial mixing, but without coalescing the aqueous layers. Impressively, this process “can perform the separation of metals in a matter of minutes, using a low concentration of extracting agents and with high selectivity”, according to co-author Dr. Cristóbal QUINTANA. This study demonstrates that concentric liquid reactors, and especially their segmented versions, can rapidly separate valuable metals from highly concentrated mixtures using much lower concentrations of extractants than in existing methods and can access unexplored ranges of process parameters. These aspects, as well as favorable power-to-operate vs. reactor-size scaling, make concentric liquid reactors an interesting alternative to the traditional hydrometallurgical methods and potentially applicable to the separations of other valuable metals. Professor Grzybowski explains, “The technology is also forward-looking in the sense that, as we show, it is tunable to different "feed" metal compositions and of course, to metals other than those used in batteries.”
Notes for editors
- Reference
- Media Contact
- About the Institute for Basic Science (IBS)
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20