주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| Title | One electrode fits all functional groups | ||
|---|---|---|---|
| Embargo date | 2020-10-09 03:00 | Hits | 585 |
| Research Center |
Center for Catalytic Hydrocarbon Functionalizations |
||
| Press release | |||
| att. | |||
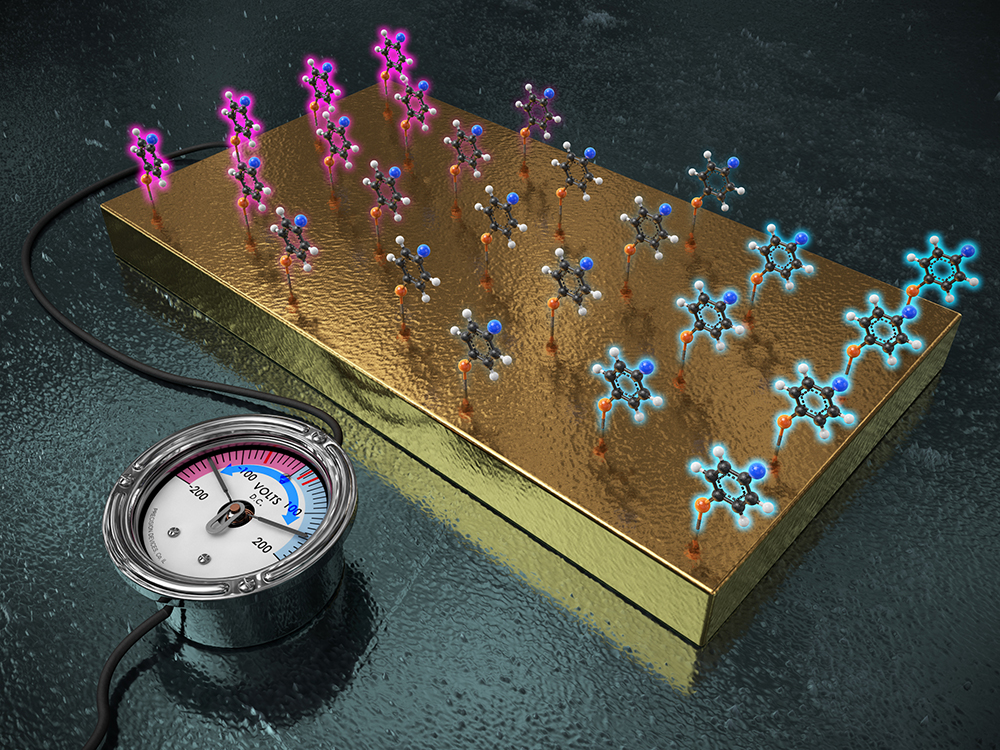
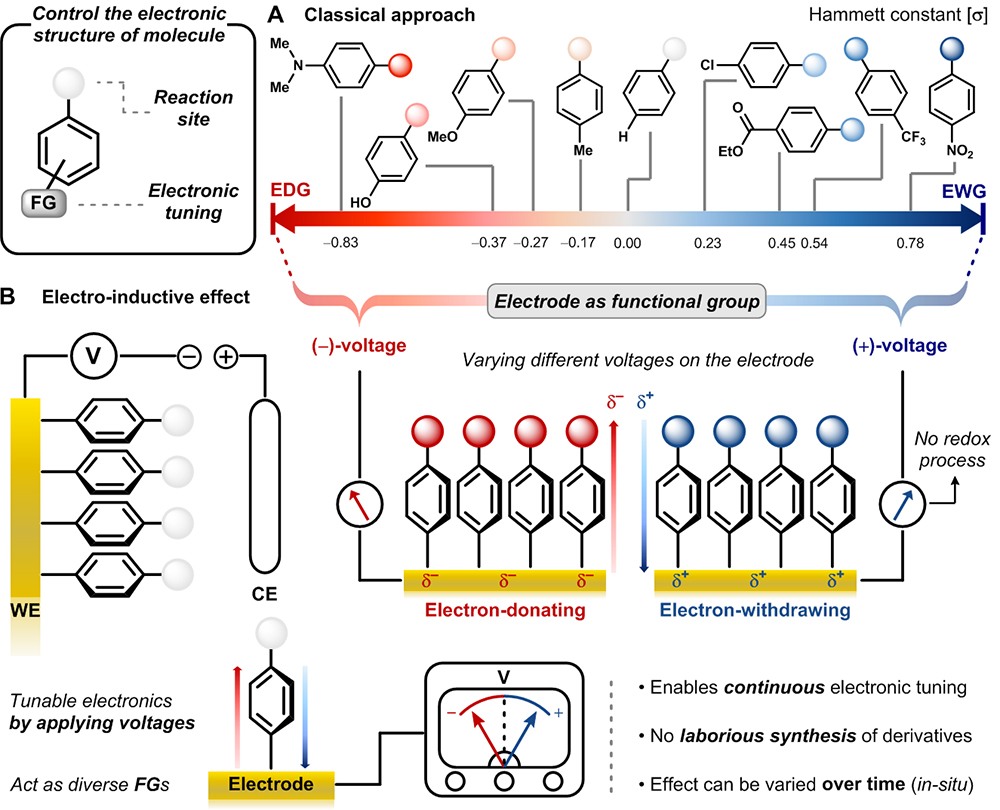
One electrode fits all functional groupsSwitching voltage of an electrode alone can fine-tune the reactivity of a molecule. As we learned in chemistry class, chemical reaction occurs with the formation or cleavage of bonds between atoms. These chemical bonds form when atoms share or exchange electrons. The chemical reactivity can be controlled in several ways. Among them, the control of electronic property at the reaction site is generally employed. For example, electron-rich molecule prefers to react with a molecule that can readily accept electrons. Many atoms can form ‘functional groups’ that either donate or withdraw electrons and control the electron density distribution of a molecule. These functional groups can vary the electronic property of the molecule to speed up the intended chemical reaction. Commonly referred to as “inductive effect”, the electron-donating group pushes electrons to increase the electron density at the site where the reaction takes place. Conversely, electron-withdrawing group removes electrons and reduces the electron density of the reaction site. In 1937, the American chemist Louis P. Hammett quantified the electronic effect of functional groups in various types of organic reactions. More than 80 years later, Hammett’s research is still widely used to design an efficient reaction or catalysis by modulating the electronic property of target molecules. However, this approach involves a frustrating and inefficient amount of tasks. Since each functional group can render only a specific inductive effect and sometimes, it may not be possible to synthesize target molecules which have desired functional groups due to a synthetic difficulty. These constraints have forced researchers to prepare numerous derivatives for a series of complicated reaction scenarios.
IBS and KAIST researchers employed the gold electrode and attached the target molecules onto the electrode. Just like functional groups generate diverse electronic effects, one electrode fits all reactions as the single electrode can behave like multiple functional groups just with the switch of applied voltage. The application of (+) voltage on the electrode decreased the electron density at the reaction site. Conversely, when (–) voltage was applied, the electrode acted as an electron-donating group, increasing the electron density at the reaction site.
Led by professor BAIK Mu-Hyun [the Center for Catalytic Hydrocarbon Functionalizations within the Institute for Basic Science (IBS) & the Department of Chemistry within the Korea Advanced Institute of Science and Technology, KAIST in Daejeon, South Korea] and HAN Sang Woo (the Department of Chemistry within the Korea Advanced Institute of Science and Technology, KAIST) a research team at the IBS and KAIST discovered what they call the “electro-inductive effect” offering an alternative to the classical approach of chemical synthesis. Employing a gold electrode, the researchers attached the target molecules onto the electrode. “Just like functional groups generate diverse electronic effects, we wished to change the properties of the immobilized molecules using different voltages. The beauty of our discovery is that one electrode fits all reactions as the single electrode can behave like multiple functional groups just with the switch of applied voltage”, notes professor Mu-Hyun Baik.
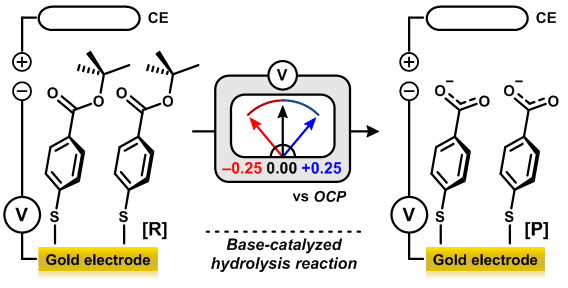
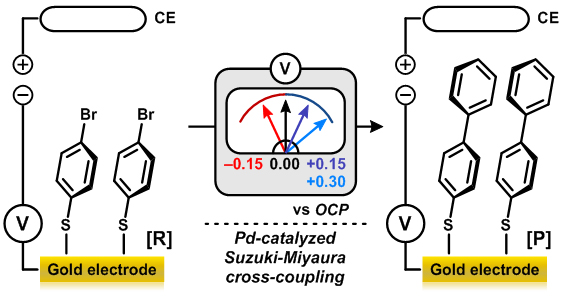
The research team showed that the gold electrode can serve as a “universal functional group” to control the reactivity of a molecule, thus propelling or inhibiting the reaction just by switching the voltage applied to the electrode. In the “base-catalyzed ester hydrolysis reaction”, a representative organic reaction that Hammett had used to establish the conventional inductive effect 80 years ago, the application of (+) voltage on the electrode decreased the electron density of the carbonyl carbon in the ester molecule. Thus, the reactivity of the ester molecule was significantly enhanced. Conversely, when (–) voltage was applied, the reaction barely proceeded owing to the high electron density of the reaction site. (Figure 2-1) They have also successfully controlled the reaction rate of the “Suzuki-Miyaura cross-coupling reaction”, a major catalytic method for forming carbon-carbon bonds. (Figure 2-2) It is of great significance that the electrode can control the reactivity not only in the organic reaction, but also in the complicated organometallic catalytic reaction.
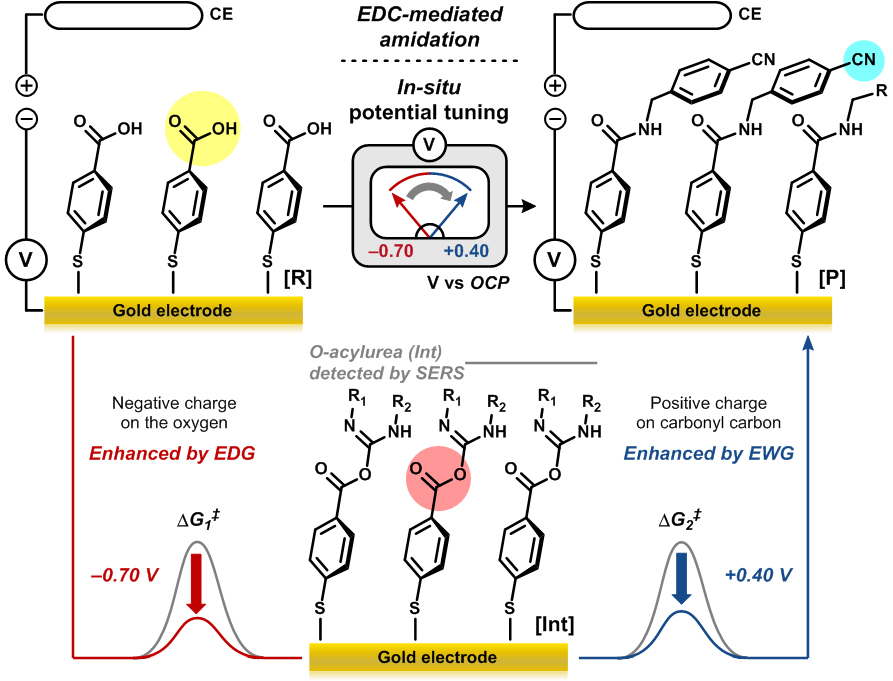
The researchers also confirmed that this “universal function group” allows for fine-tuning of the electronic property and reactivity of the molecule in the middle of a chemical reaction. (in-situ tuning). EDC [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide]-mediated amidation reaction can be divided into two steps: The first step is accelerated by the electron-donating group, whereas the second step is enhanced by the electron-withdrawing group. EDC-mediated amidation reaction hardly proceeded when one type of voltage was applied. (Figure 3) The “universal functional group” successfully changed the reactivity of the molecule while the reaction was in progress. The idea of covalently attaching molecules to an electrode is not new. However, most of such cases involve redox reactions. “The chemical reactions controlled by the electro-inductive effect do not involve redox processes. The electrode only serves as a functional group with tunable inductive effect. In addition, this study explored how the electro-inductive effect works in actual chemical reactions,” explains professor Mu-Hyun Baik. As researchers confirmed the potential of replacing functional groups with a gold electrode at different voltages, they are going to employ other types of electrodes, such as carbon-based electrodes to ensure a practical and scalable application of this study. Notes for editors - References - Media Contact: - About the Institute for Basic Science (IBS): |
|||
|
|
|||
| Next | |
|---|---|
| before |
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20