주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
| Title | Righty or Lefty, As You Like | ||
|---|---|---|---|
| Embargo date | 2019-02-21 16:16 | Hits | 1433 |
| Press release | |||
| att. | |||
Righty or Lefty, As You LikeNew iridium catalyst enables selective synthesis of valuable drug ingredients
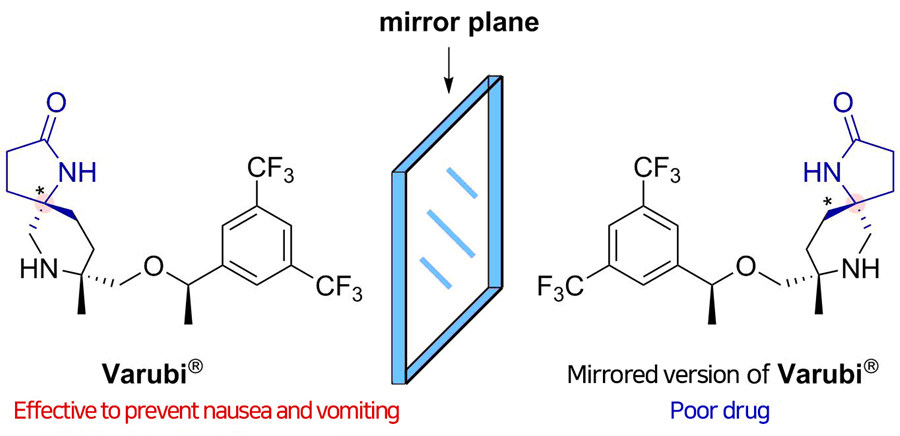
Did you know that more than half of the drugs currently in use are chiral, potentially resulting in two different responses in the body? Chiral compounds are pairs of molecules that are mirror images of each other, just like a person's right and left hand. Since the body interacts with each molecule differently, a chiral drug may produce the desired effects from one molecule, along with an unwanted side effect from its mirror image. It would be much safer and efficient to make pure compounds without forming their unnecessary mirrored versions that will go to waste, but this selective synthesis has remained one of the most difficult challenges in drug discovery.
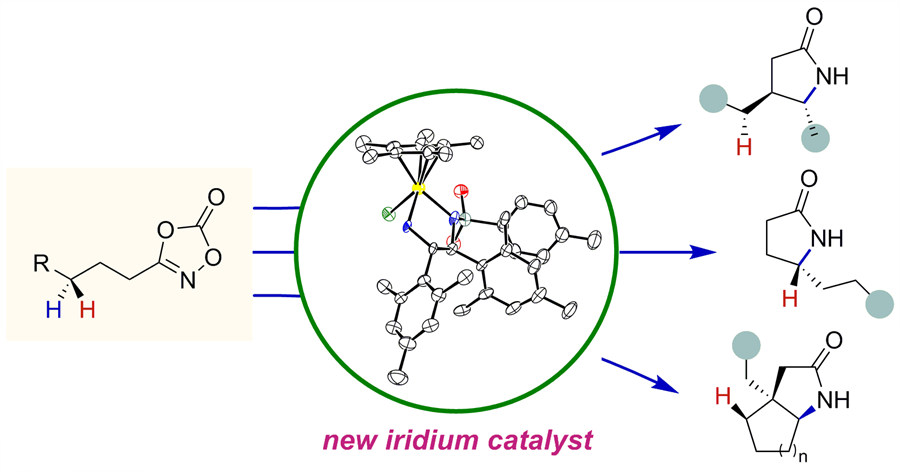
Led by professor Chang Sukbok, researchers at the Center for Catalytic Hydrocarbon Functionalizations within the Institute for Basic Science (IBS) in Daejeon, South Korea reported that they created a group of new chiral iridium catalysts for the selective synthesis of chiral lactams. Though lactams are key building blocks in many pharmaceutical agents, for example penicillin – a notable lactam-based antibiotic, there have been few efficient ways to single out only one molecule from a pair of the symmetric mirror images. The new catalysts allowed the researchers to bias synthesis in favor of producing one product over the other with 99 percent selectivity.
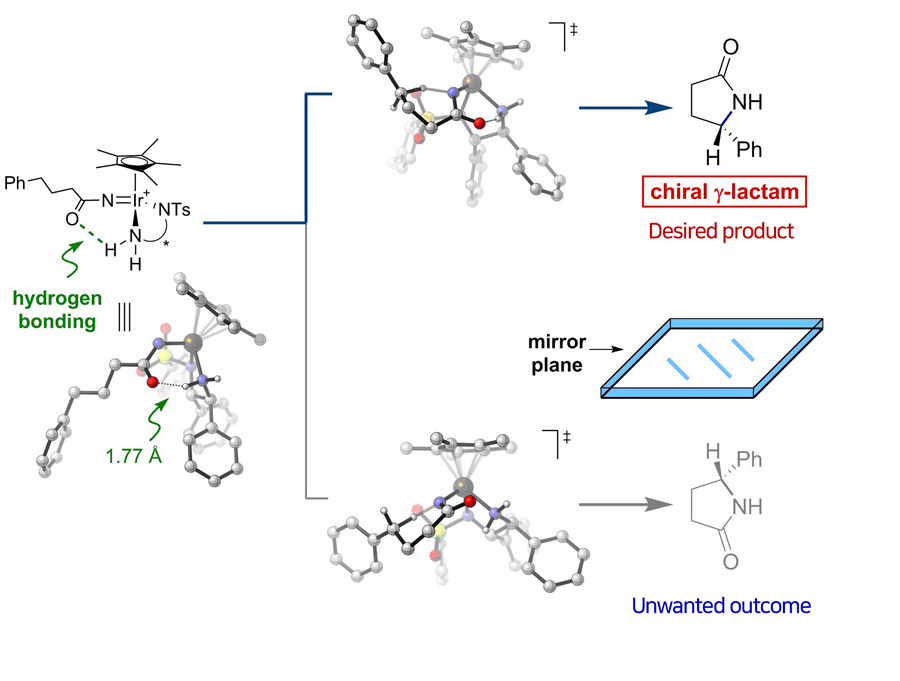
The atoms in chiral drugs are connected in the same way but differ in how they are oriented in space. The researchers identified which catalyst induced a righty rotation and which caused a lefty rotation. Combining experimental observations and detailed computer simulations, they found that transient formation of hydrogen bonding between a substrate and the catalyst induced such high segregation in the reaction.
The hydrogen bonding is a ubiquitous interaction of molecules in nature. As this connection stabilizes intermediates and transition states during the course of a reaction, it eventually leads to the production of chiral lactams allowing high selectivity. The first author, Dr. Yoonsu Park states, "It was key to understand the reaction mechanism in order to facilitate further catalyst development. This mechanism-based approach and reaction design model allowed us to find this novel transformation."
Notably, this study suggests a sustainable protocol to produce highly sought-after, invaluable chiral lactams. By using inexpensive and readily available feedstock hydrocarbons, the researchers produced a group of chiral lactams in different shapes. As their chirality and diverse structures enable lactams to function as an active compound in the body for antibiotic, anti-inflammatory, or anti-tumoral functions, this study may facilitate the development of potential drugs in a more efficient and cheaper way. "This is a major step forward in the area of asymmetric catalysis. As lactams find several applications in medicinal, synthetic, and material chemistry, our study may offer foundations in developing safer and more effective clinical drugs," explains professor Chang. Dahee Carol Kim Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
|
|
|||
| Next | |
|---|---|
| before |
- Content Manager
- Communications Team : Kwon Ye Seul 042-878-8237
- Last Update 2023-11-28 14:20