주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
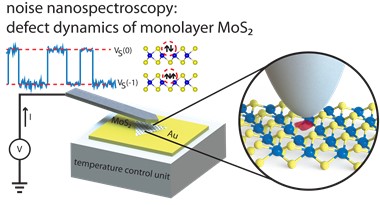
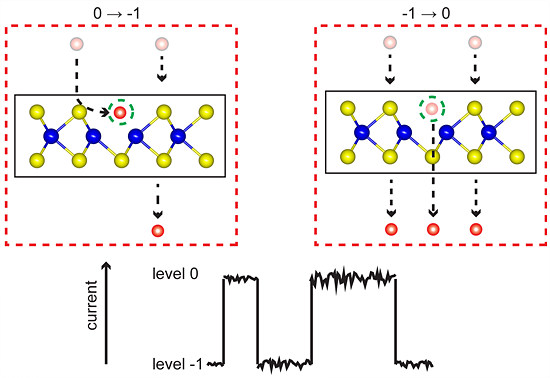
Understanding the Impact of Defects on the Properties of MoS2- Highly desired in the petrochemical industry, but generally unwanted in electronics manufacture, defects in MoS2 influence the properties and utility of this material. Analysis of atomically thin MoS2 reveals how defects behave and relate to MoS2’s anomalies - Researchers at the Center for Integrated Nanostructure Physics, within the Institute for Basic Science (IBS), have shown that defects in monolayer molybdenum disulfide (MoS2) exhibit electrical switching, providing new insights into the electrical properties of this material. As MoS2 is one of the most promising 2D semiconductors, it is expected that these results will contribute to its future use in opto-electronics. Defects can cause major changes in the properties of a material, leading to either desirable or unwanted effects. For example, petrochemical industry has long taken advantage of the catalytic activity of edges of MoS2, caused by the presence of a high concentration of defects, to produce petroleum products with reduced sulfur dioxide (SO2) emissions. On the other hand, having a pristine material is a must in electronics. Currently, silicon rules the industry, because it can be prepared in a virtually defect-free manner. In the case of MoS2, its suitability for electronic applications is currently limited by the presence of naturally occurring defects. So far, the precise link between these defects and the degraded properties of MoS2 has been an open question. In IBS, a team of physicists, material scientists, and electrical engineers worked closely together to explore the electronic properties of sulfur vacancies in MoS2 monolayers, using a combination of atomic force microscopy (AFM) and noise analysis. The scientists used a metallic AFM tip to measure the noise signal, i.e., the variation of electrical current passing through a single layer of MoS2 placed on a metal substrate. The most common defects in MoS2 are instances of missing single sulfur atoms, also known as sulfur monovacancies. In a perfect sample, each sulfur atom has two valence electrons that bind to two molybdenum electrons. However, where a sulfur atom is missing, these two molybdenum electrons are left unsaturated, defining the neutral state (0 state) of the defect. However, the team observed rapid switching events in their noise measurements, indicating the state of the vacancy switched between neutral (0 state) and charged (-1 state). “The switching between 0 and -1 is happening continuously. While an electron resides at the vacancy for a while, it is missing from the current, such that we observe a current drop,” explains Michael Neumann, one of the co-first authors of the study. “This goes a long way towards understanding the known anomalies of MoS2, and it is very interesting that sulfur vacancies alone are enough to explain these anomalies, without requiring more complex defects." According to the experiments and earlier calculations, two electrons can be also trapped at the vacancy (-2 state), but this does not seem to be energetically favored.
The new observation that sulfur vacancies can be charged (-1 and -2 states) sheds light on several MoS2 anomalies, including its reduced electron mobility observed in MoS2 monolayer samples: electrons move following the direction of an applied voltage, but get scattered by charged defects. “The -1 state is occupied around 50% of the time, which would lead to scattering of electrons, and thus explain why MoS2 has such poor mobility,” clarifies Neumann. Other MoS2 characteristics which can be explained by this study are the n-type doping of MoS2, and the unexpectedly large resistance at the MoS2-metal junction. "This research opens up the possibility of developing a new noise nanospectroscopy device capable of mapping one or more defects on a nanoscale scale over a wide area of a 2D material," concludes the corresponding author Young Hee Lee. The full study is available on Nature Communications. Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Integrated Nanostructure PhysicsPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20