주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
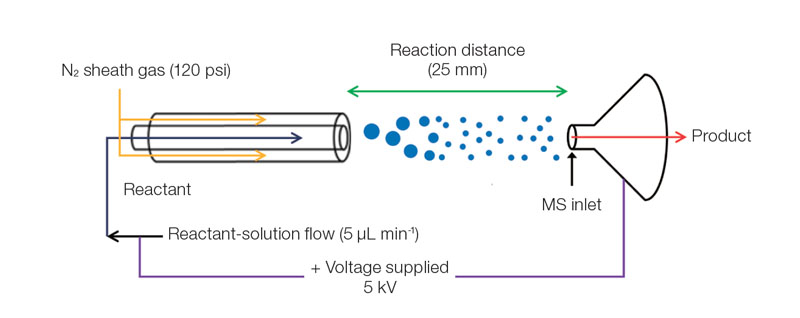
Origin of life found in micro-sized water drops- Spontaneous sugar phosphorylation occurs outside living organisms - Every living organism engages in metabolism, a process by which substances are absorbed, synthesized and degraded for various uses to sustain life. This process, encompassing all chemical changes that occur throughout the lifetime of organisms, requires energy generated through the phosphorylation reactions of sugars. Essential components that make life possible – for example, ribonucleic acid, (RNA), deoxyribonucleic acid (DNA) and adenosine triphosphate (ATP) – are the products of phosphorylation reactions, or the addition of phosphoryl groups to substances. Though in vivo phosphorylation occurs spontaneously using ATP and reaction-specific enzymes, it is not a spontaneous reaction thermodynamically, known to occur only within cells. Explaining the mechanisms of intracellular phosphorylation, therefore, has been the first step to cracking the mystery of the origin of life. Researchers at the IBS Center for Plant Aging Research (Director NAM Hong Gil) worked with a research team from Stanford University and discovered that ribose, glucose and other sugars are spontaneously phosphorylated at accelerated speeds inside micrometer-sized water drops. Their observation has shaken the conventional view – such reactions do not spontaneously occur in an aqueous solution under lab environments – and shed new light on investigating life phenomena by triggering a vital life process, phosphorylation, outside living organisms.
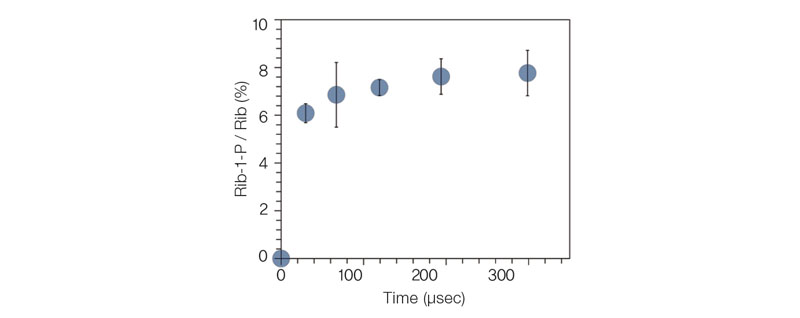
T he occur rence of diver se metabolic reactions is confined to intracellular space inside of an organism. In order to capture a phosphorylation reaction occurring in physically cell-like environments, the researchers chose micrometer-sized droplets and observed that sugar phosphates are produced there within a few microseconds. Unlike in bulk solution, the entropy in the droplets does not decrease; the surface electrical field of the droplets alters the material orientation and reaction energy, which presumably made the reaction possible.
The team’s discovery indicates that the first life may have sprung from a water drop containing small, simple molecules. Through previous research, the team already demonstrated that micrometer-sized aqueous droplets are favorable conditions for diverse chemical reactions, where their reactions rates are accelerated 1000 to 1 million-fold. As ribose 1-phosphate, one of the sugar phosphates produced in the water drops, is a basic building block of RNA’s nucleobases, the research has suggested a possibility of these microdroplets as a synthetic pathway for nucleobases and RNA. These results are expected to deepen the understanding of metabolism and provide a reliable explanation on how life began and why it has to end. |
|||
Center for Plant Aging ResearchPublication Repository |
| before | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20