주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
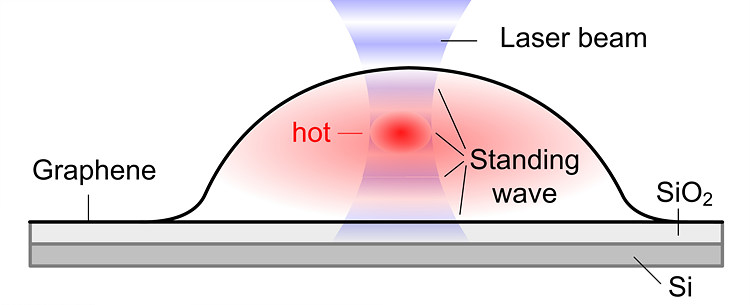
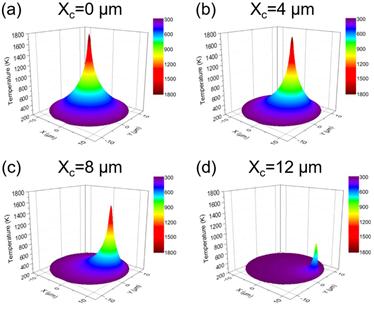
Bubbly Graphene: How Cool or Hot Are You?- In a perfect match between theory and experiments, IBS scientists predict, measure and control the temperature of large graphene bubbles with a single laser beam - A team of researchers at the Center for Multidimensional Carbon Materials, within the Institute for Basic Science (IBS) have measured and controlled the temperature of individual graphene bubbles with a single laser beam for the first time. The study is now available from Physical Review Letters. The highly elastic and flexible nature of graphene allows for the creation of stable large bubbles, in a more or less controlled fashion. The strain and curvature introduced by the bubbles is known to tune the electronic, chemical, and mechanical properties of this material. Generally, graphene bubbles are more reactive than flat graphene, so they might be more prone to be decorated with chemical groups. Bubbles might serve as tiny, closed reactors, and their curved surface could provide a lens effect. Understanding how temperature varies within bubbles is an important factor for several applications. “If you think that chemical reactions could be carried out inside the bubble or on the surface of each graphene bubble, then changing the temperature distribution in a bubble will significantly influence reactions taking place,” says Yuan Huang, the first author of the study. In this study, bubbles are formed at the interface between a graphene sheet and a silica (SiO2/Si) substrate it lies on. The SiO2 surface attracts some molecules that evaporate when heated, creating bubbles. As also predicted by the theorists of the team, Xiao Wang and Feng Ding, the temperature oscillates with the bubble height. Although each bubble is only several micrometers in width and about one micrometer in height, the scientists could detect a variation in temperature, not only between the center and the edges, but also at different heights of the bubble.
When a graphene bubble is illuminated with a laser beam, incident and reflected rays overlap forming an optical standing wave on the surface. Increasing the laser power has the effect of selectively heating specific regions of the bubble, which correspond to the maximum interference of the standing optical wave. IBS scientists detected local changes in temperature within each bubble using Raman spectroscopy, a standard technique to measure graphene characteristics and morphology. “Standing waves near surfaces have been ignored for a long time and have only rarely been observed in a direct manner. The results are surprising. The laser beam can efficiently heat the graphene, and we can determine the thermal conductivity in graphene bubbles from its temperature distribution,” explains Wolfgang Bacsa, one of the members of the team, and visiting scientist from CEMES-CNRS and University of Toulouse in France.
“These results confirm the high thermal conductivity of graphene previously measured, demonstrate the excellent adhesion around the perimeter of the graphene bubble, and provide new perspectives on how to heat graphene bubbles on specific locations,” concludes Rod Ruoff, coauthor and director of the Center for Multidimensional Carbon Materials. “The more we know about the physical properties of graphene bubbles, the more we might be able to make use of them in different ways.” For example, an intriguing application could be the creation of graphene sheets with circular holes, like a ‘polka dot’ pattern. As overheating of the bubbles causes them to burst, the pores decorated with specific chemical groups could work as molecular selective filters. Graphene’s unique properties never cease to amaze. Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Multidimensional Carbon MaterialsPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20