주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Theoretical Physics of the Universe(Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe(Cosmology, Gravity and Astroparticle Physics Group)
- Center for Exotic Nuclear Studies
- Center for Artificial Low Dimensional Electronic Systems
- Center for Underground Physics
- Center for Axion and Precision Physics Research
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Van der Waals Quantum Solids
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research(Neuro Technology Group)
- Center for Neuroscience Imaging Research(Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
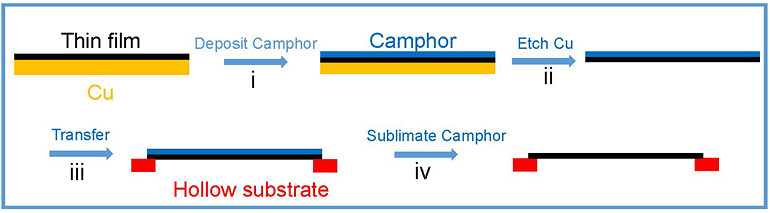
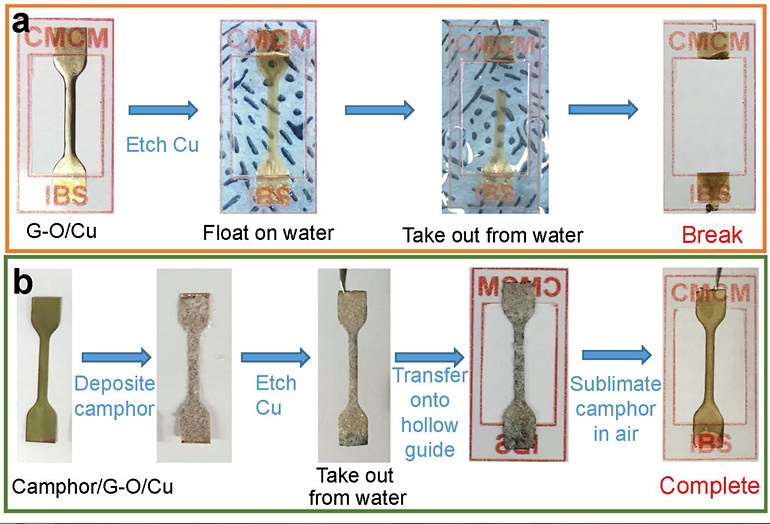
Mechanical Stiffness and Strength of Centimeter-Scale Atom Thick Graphene- IBS scientists have measured the tensile strength of centimetre-scale monolayer graphene for the first time, using camphor as a naturally-volatilizing support - Graphene is one of the stiffest and thinnest materials in the world. However, the mechanical properties of monolayer graphene pieces bigger than a few micrometers have never been tested, simply because moving such an ultrathin film to a standard testing apparatus has not been possible. Researchers at the Center for Multidimensional Carbon Materials, within the Institute for Basic Science (IBS), have managed to measure the tensile strength of centimeter-scale monolayer graphene films, using camphor - a chemical that easily volatilizes at room temperature - as a temporary support layer. Published in Advanced Materials, this trick could be applied to transfer and study other challenging 2D materials. Recently, new and impressive characteristics of ultrathin films have been unveiled, with promising applications in microelectromechanics, optoelectronics, and biology. For example, the combination of graphene’s transparency, impermeability, conductivity, and elasticity could be used for flexible electronics, transparent protective coatings, and barrier films. Measuring the tensile strength of materials involves pulling samples until they break. However, testing 2D materials is a complex task. Traditionally, a layer of another material (substrate) is used as a “tray”, to help with the transfer and to give these ultrathin films some support. Such substrates have pros and cons: there is the risk of damaging the film when the substrate is peeled away, or if the substrate layer is too thick, distinguishing the properties of the substrate from the ones of the 2D material of interest becomes impossible. In this study, camphor is used as a transient support, and what differentiates it from conventional methods is that it is sublimed away in air at room temperature naturally, or at higher temperatures for faster processing. Thanks to this method, ultrathin films with an area larger than 1 cm x 1 cm are transferred without damage, then the camphor layer disappears in the air without leaving traces. In this way, tensile measurements were made on centimeter-scale 300 nm-thick graphene oxide film specimens, almost ten times thinner than previously reported. It was also possible to work with a graphene oxide film that was only 35 nm thick, and suspend it over a 1 cm x 1 cm hole.
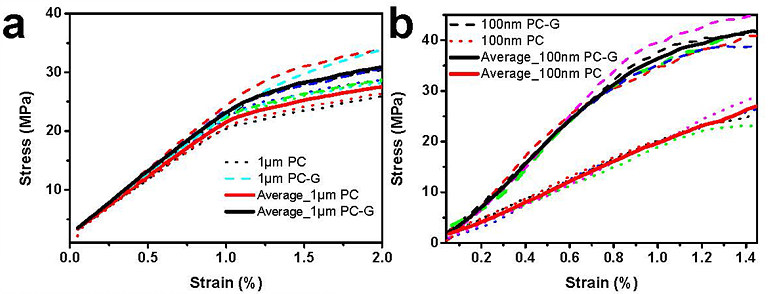
Moreover, the IBS researchers tested the new camphor-method with one-atom-thick graphene films. In this case, stretching a freestanding monolayer 2D film is not yet possible, and a polycarbonate (PC) film is used as a support layer. Without camphor, the thinnest PC/monolayer graphene assembly tested was around 1 μm thick, and graphene’s mechanical properties could not be obtained because the thick PC film dominates the response. However, IBS scientists managed to distinguish monolayer graphene properties using the camphor method and a 100 nm thick PC substrate.
The tensile tests demonstrated that these large-scale samples do have high stiffness. The team measured graphene mechanical properties in terms of the Young’s modulus, which characterizes the intrinsic stiffness of a material. The study reports that centimeter-scale polycrystal monolayer graphene has a Young’s modulus of 637-793 GPa, but that single-crystal graphene had high-end values at or near 908 GPa. As a matter of comparison, a block of high strength steel has a Young’s modulus of about 200 GPa, but accounting for the higher density of steel, that is, corrected for weight, the intrinsic stiffness of large-scale graphene is about 20 times higher. “I think this method might become a standard around the world for testing single layer 2D materials, including graphene at length scales meaningful for applications,” notes Rod Ruoff, director of the IBS Center for Multidimensional Carbon Materials. Now that the team has this technique in hand, their next challenge is to produce graphene sheets that are not only astonishingly stiff, but also incredibly strong. “Strong carbon-carbon bonds confer graphene with an impressive ideal strength, which is however lowered if there are defects in its structure,” explains first author Bin Wang. “The ideal strength of graphene is about 130 GPa, second only to the ideal strength of diamond along a particular crystallographic direction (240 GPa). But these are calculated strengths, and are never obtained for “large” specimens, like a centimeter or larger—because such specimens always have defects, also called flaws, like small cracks. Now that we have this new method for measuring large graphene specimens, we can begin to understand why the strength for our graphene samples, even the single crystal graphene samples, is typically around 8 GPa, and not higher. Understanding this will provide us a “road map” to make graphene that is much stronger—that breaks at a much higher value of percent elongation,” continues Ruoff. “And when we do that—that will change the world.” Letizia Diamante Notes for editors - References - Media Contact - About the Institute for Basic Science (IBS) |
|||
Center for Multidimensional Carbon MaterialsPublication Repository |
|||
|
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20