주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
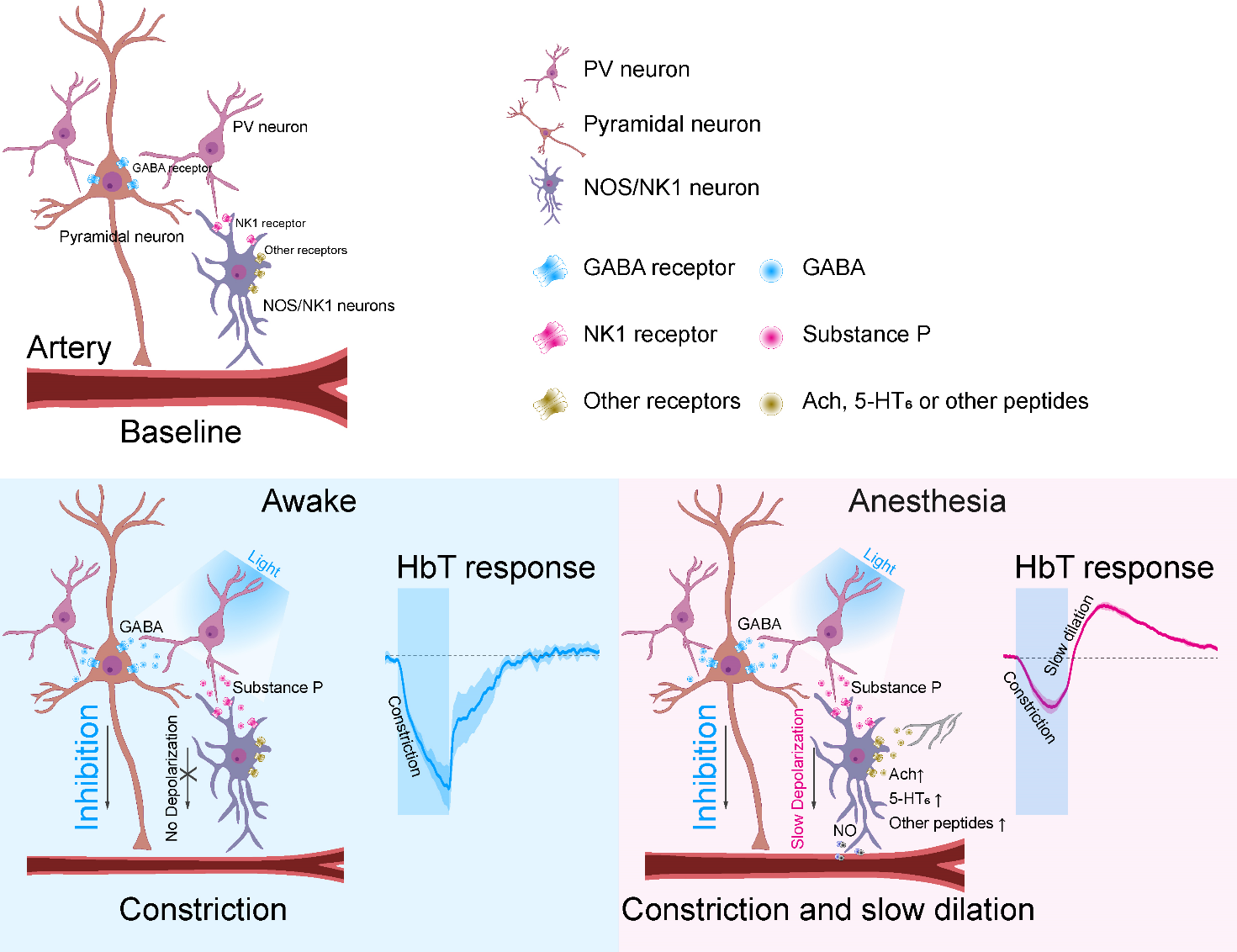
How the brain controls blood flow during sleep- “Substance P” secreted by parvalbumin interneurons drive slow vasodilation - Even while we are asleep, the brain does not rest completely. Surprisingly, the blood flow in a sleeping brain can be greater than when it is in a wakeful state. This allows the brain to remove waste metabolites, which is important to prevent the development and progression of neurological dysfunctions such as dementia. However, the exact mechanism of how the sleeping brain increases blood flow has not been known. Researchers led by Director KIM Seong-Gi of the Center for Neuroscience Imaging Research within the Institute for Basic Science, South Korea, recently discovered the secrets behind this phenomenon. It was discovered that a type of inhibitory neuron called ‘parvalbumin (PV) neuron’ secretes material called ‘substance P’ that is responsible for vasodilation and control of blood flow to the brain. Unlike other inhibitory subtype neurons, it was previously thought that GABAergic PV neurons do not release vasoactive substances, hence their role in blood flow regulation has been controversial. To investigate the role of PV neurons on blood flow regulation, the researchers expressed an opsin protein called channelrhodopsin-2 (ChR2) in mouse PV neurons, and selectively activated PV neurons by light stimulation. Vascular responses to the activation of PV neurons were measured by wide-field optical imaging and functional magnetic resonance imaging (fMRI). In addition, to identify the role of the PV neurons on blood flow under anesthesia, the researchers used ketamine/xylazine to put the animals to sleep. The results showed that in lightly anesthetized animals, stimulation of PV neurons induced vasoconstriction and a decline in blood flow. This was followed by a slow vasodilation and an increase in blood flow from 20 seconds up to a minute after cessation of stimulation. On the other hand, in active animals, the PV neuron activity only resulted in a reduction of blood flow. This means that the PV neurons have two different mechanisms for the control of cerebral blood flow depending on whether the brain is awake or asleep. Furthermore, the researchers also discovered the mechanism behind the observed ‘slow vasodilation after optogenetic stimulation. When the PV neurons are activated, it inhibits the nearby excitatory neurons, which causes vasoconstriction and reduced blood flow. At the same time, it was found that these PV neurons release a peptide called ‘substance P’, which is responsible for the observed slow vasodilation. Substance P activates cells called nitric oxide synthase (nNOS) GABAergic neurons secreting nitrous oxide, a known vasodilator. The present research finally unveiled the factors that control blood flow to the brain during sleep, and the previously unknown role of PV neurons in this process. Director Kim said, “Our research suggested a new direction of research on cerebral blood flow control mechanisms, with possible implications in insomnia and sleep disorder treatment.” This research was conducted jointly with Professor SUH Minah’s team at the SungKyunKwan University (SKKU) Department of Biomedical Engineering and Professor Patrick J. DREW of the Pennsylvania State University.
Notes for editors
- Reference
- Media Contact
- About the Institute for Basic Science (IBS)
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20