주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Center for Memory and Glioscience (Cognitive Glioscience Group)
- Center for Memory and Glioscience (Learning and Memory Group)
- Center for Synaptic Brain Dysfunctions
- Center for RNA Research
- Center for Genomic Integrity
- Center for Vascular Research
- Center for Genome Engineering
- Center for Microbiome–Body–Brain Physiology
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Center for Memory and Glioscience (Cognitive Glioscience Group)
- Center for Memory and Glioscience (Learning and Memory Group)
- Center for Synaptic Brain Dysfunctions
- Center for RNA Research
- Center for Genomic Integrity
- Center for Vascular Research
- Center for Genome Engineering
- Center for Microbiome–Body–Brain Physiology
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
How the Brain Distinguishes Between Pain and Itch- Modality-specific neurons in the anterior cingulate cortex play a key role in sensory discrimination - A research team led by KAANG Bong-Kiun, director of the Center for Cognition and Sociality within the Institute for Basic Science (IBS), and KO Hyoung-Gon, professor at Kyung Hee University College of Dentistry, have uncovered the neural mechanisms underlying the processing of pain and itch in the anterior cingulate cortex (ACC). This study provides new insights into how the brain distinguishes between these two distinct sensory experiences. Pain and itch are both unpleasant sensations, but they trigger different responses—pain often prompts withdrawal, while itching leads to scratching. Until now, scientists have struggled to understand how the brain processes these sensations separately, as they share overlapping neural pathways from the spinal cord to the brain. Both stimuli are transmitted from the spinal cord to the thalamus and brainstem, eventually reaching the ACC. The ACC is a key brain region involved in various functions, ranging from basic sensory processing to higher-order cognition. However, a comprehensive understanding of how a limited number of neurons within the ACC execute such diverse functions has been lacking. This study provides fundamental insights into how ACC neurons selectively process pain and itch information. By analyzing neuronal response patterns in the ACC to pain and itch stimuli, the research team identified two distinct neuronal populations: 1. Non-selective neurons, which respond to both pain and itch stimuli indiscriminately. 2. Stimulus-specific neurons, which were selectively activated by either pain or itch stimuli. Furthermore, using the dual-eGRASP technique—an advanced synaptic analysis method developed by Kaang’s research team (Science, 2018)—the researchers discovered that stimulus-specific neurons in the ACC receive distinct synaptic inputs from the mediodorsal thalamus (MD). This finding indicates that pain and itch are processed by independent neuronal populations within the ACC, which receive differentiated synaptic inputs, providing fundamental insights into the neural mechanisms of pain and itch processing. To further confirm the role of these neurons, the team used chemogenetic techniques to selectively deactivate either pain-specific or itch-specific neurons. The results showed suppressing pain neurons reduced pain perception without affecting itch, and vice versa. This discovery suggests that these neurons play a direct role in shaping how we experience pain and itch. This study presents a groundbreaking discovery that the role of ACC neurons in processing pain or itch is predetermined. Importantly, the study demonstrates that pain- and itch-specific neurons in the ACC are synaptically paired with corresponding stimulus-specific neurons in the MD, establishing independent neural circuits for pain and itch processing. These findings challenge the conventional assumption that pain and itch signals follow overlapping pathways and instead highlight distinct neural mechanisms for each sensation. Given that the ACC is known to mediate the affective aspects of pain and itch, this study suggests that separate neuronal populations are responsible for encoding the subjective experience of pain and itch. Building upon these findings, the research team aims to further investigate the brain’s complex sensory processing mechanisms. Corresponding author KAANG Bong-Kiun stated, “The ACC is an important brain area not only for memory storage but also for processing higher-order emotions such as pain and conflict. Through this study, we have taken a step further in understanding emotional memory at the synaptic level.” Co-corresponding author and first author KO Hyoung-Gon commented, “I am particularly interested in how these pain- and itch-selective neural circuits change under pathological conditions. Moving forward, we plan to expand our research to explore the interactions between these circuits.” This study was published in the journal Nature Communications.
Formalin was injected into the hind paw and histamine into the neck of a mouse to induce pain and itch, respectively. The activation patterns of anterior cingulate cortex (ACC) neurons were then analyzed. To determine whether the activation patterns vary not only based on the type of sensory stimulus but also on prior stimulus experience, the experiment was designed to distinguish between primary and secondary stimuli. Green represents neurons activated by the primary stimulus. Red represents neurons newly activated by the secondary stimulus (pain or itch). The research team quantitatively compared the distribution patterns, overlap ratios, and locations of neurons activated by both stimuli, confirming that pain and itch are processed by independent neuronal populations. Additionally, some neurons responded exclusively to a specific sensory input (pain or itch), while others exhibited stimulus-independent responses influenced by prior stimulus experiences.
(a) An image showing the expression of dual-eGRASP in the anterior cingulate cortex (ACC). The middle image displays neurons activated by an itch-inducing stimulus. The right image displays neurons activated by a pain-inducing stimulus. (b) An image of dendritic spines in the ACC, where dual-eGRASP is expressed following pain and itch stimulation. Cyan-GRASP (cyan signals) represents input from mediodorsal thalamus (MD) neurons activated by an itch-inducing stimulus. Yellow-GRASP (yellow signals) represents input from MD neurons activated by a pain-inducing stimulus. Analysis of the relative proportions of dual-eGRASP signals revealed that synaptic inputs to ACC neurons are predetermined by the presynaptic neurons in the MD, indicating that the neuronal connectivity is established in advance based on the stimulus-specific neuronal populations in the MD. Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS)
|
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20










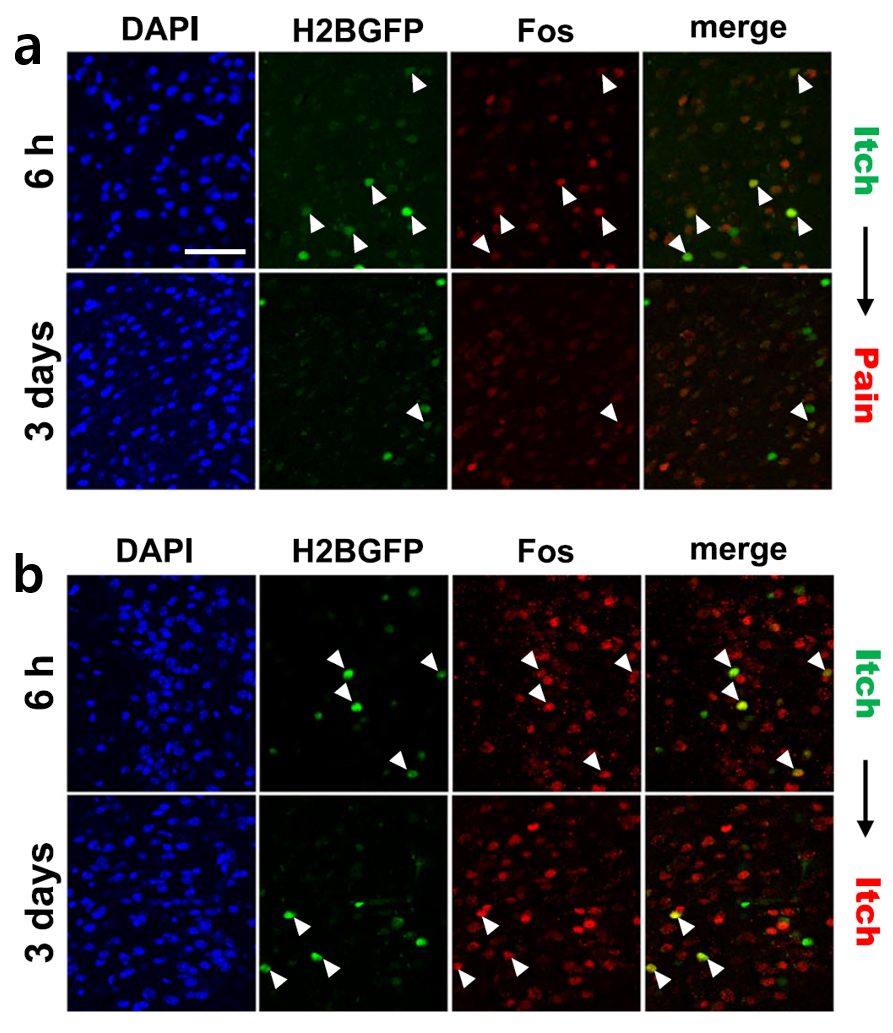 Figure 1. Visualization of Neurons Activated by Pain- and Itch-Inducing Stimuli
Figure 1. Visualization of Neurons Activated by Pain- and Itch-Inducing Stimuli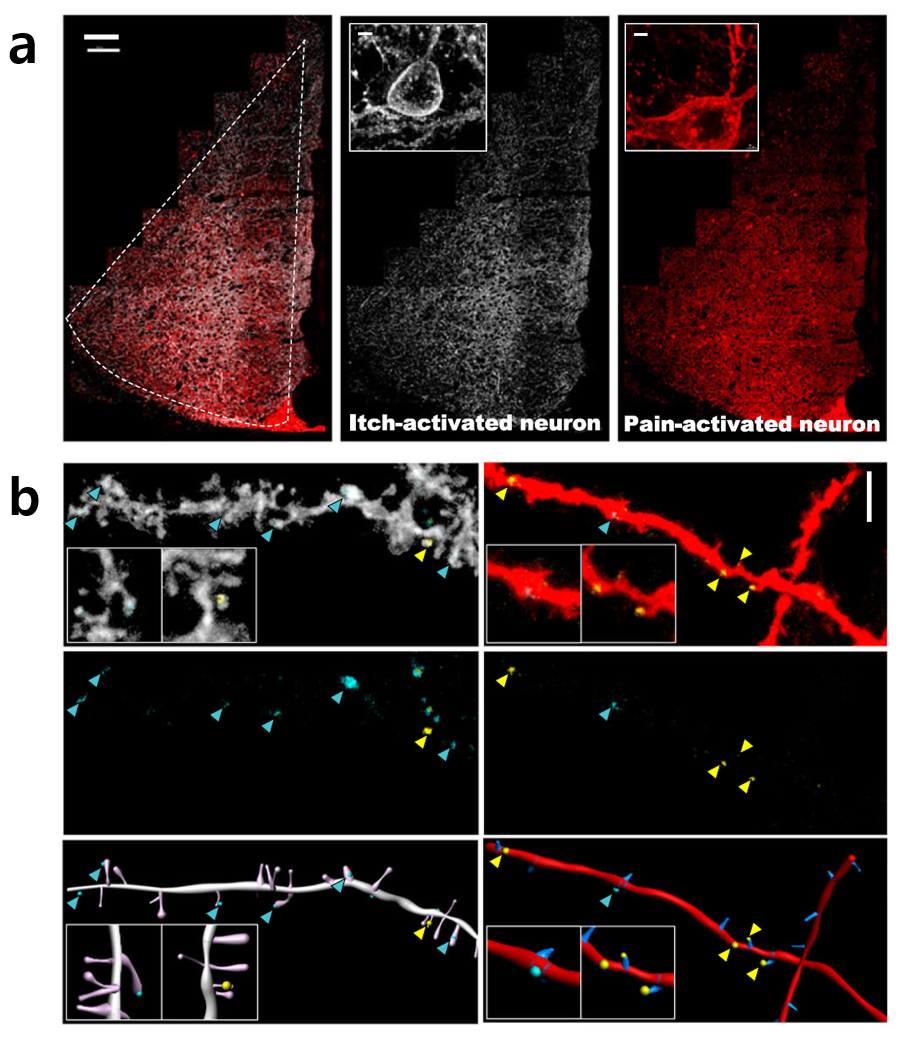 Figure 2. Synaptic Connections Between Stimulus-Specific Neuronal Populations in the ACC and MD
Figure 2. Synaptic Connections Between Stimulus-Specific Neuronal Populations in the ACC and MD

