주메뉴
- About IBS 연구원소개
-
Research Centers
연구단소개
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center 뉴스 센터
- Career 인재초빙
- Living in Korea IBS School-UST
- IBS School 윤리경영


주메뉴
- About IBS
-
Research Centers
- Research Outcomes
- Mathematics
- Physics
- Center for Underground Physics
- Center for Theoretical Physics of the Universe (Particle Theory and Cosmology Group)
- Center for Theoretical Physics of the Universe (Cosmology, Gravity and Astroparticle Physics Group)
- Dark Matter Axion Group
- Center for Artificial Low Dimensional Electronic Systems
- Center for Theoretical Physics of Complex Systems
- Center for Quantum Nanoscience
- Center for Exotic Nuclear Studies
- Center for Van der Waals Quantum Solids
- Center for Relativistic Laser Science
- Chemistry
- Life Sciences
- Earth Science
- Interdisciplinary
- Center for Neuroscience Imaging Research (Neuro Technology Group)
- Center for Neuroscience Imaging Research (Cognitive and Computational Neuroscience Group)
- Center for Algorithmic and Robotized Synthesis
- Center for Genome Engineering
- Center for Nanomedicine
- Center for Biomolecular and Cellular Structure
- Center for 2D Quantum Heterostructures
- Center for Quantum Conversion Research
- Institutes
- Korea Virus Research Institute
- News Center
- Career
- Living in Korea
- IBS School
News Center
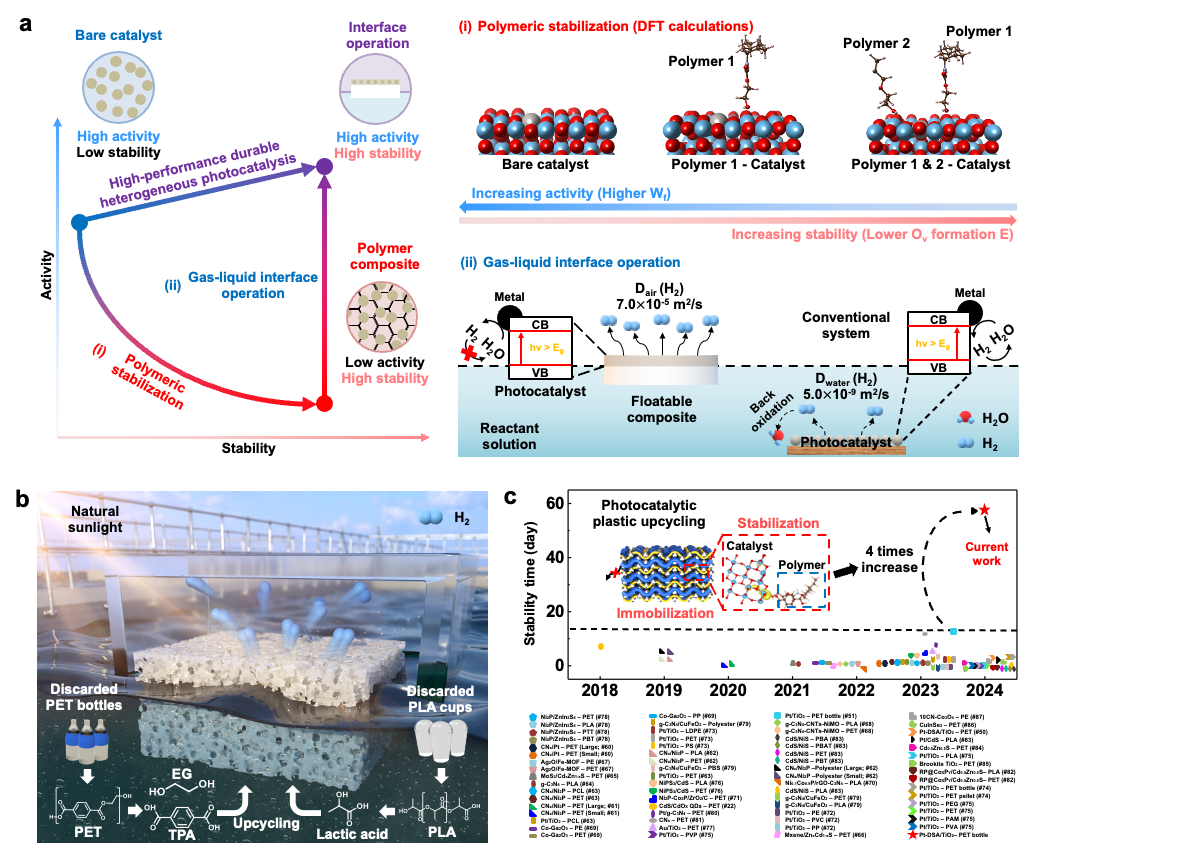
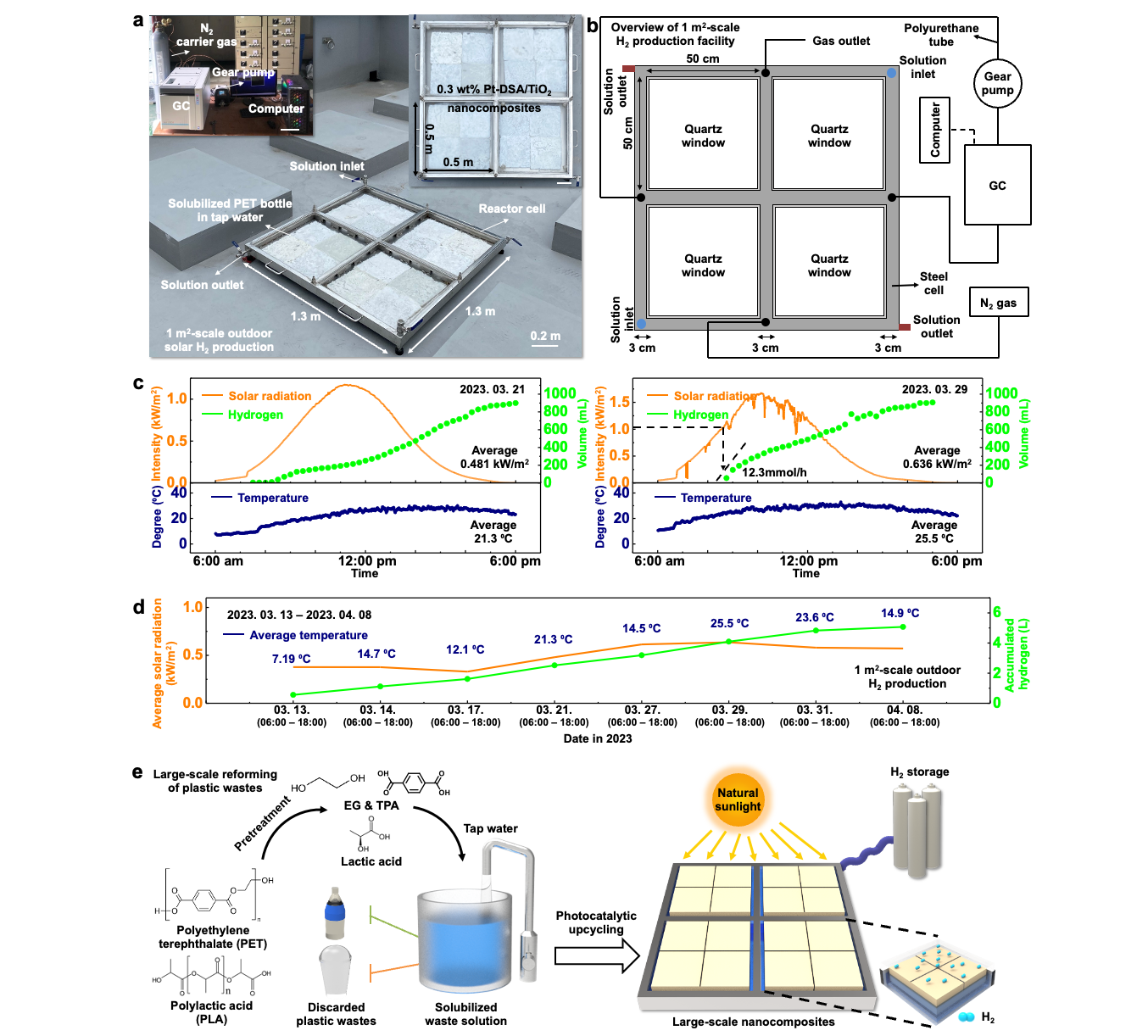
From Plastic Waste to Clean Hydrogen: A Scalable Solar-Powered Solution– Korean researchers develop eco-friendly technology to turn plastic into hydrogen using sunlight – A team of Korean scientists has developed an innovative green technology that transforms plastic waste into clean hydrogen fuel using only sunlight and water. Researchers at the Institute for Basic Science (IBS) Center for Nanoparticle Research, led by Professor KIM Dae-Hyeong and Professor HYEON Taeghwan of Seoul National University, announced the successful development of a photocatalytic system that produces hydrogen from PET bottles. The key innovation lies in wrapping the photocatalyst in a hydrogel polymer, which helps it float on water and stay active even under harsh environmental conditions. Hydrogen is gaining attention as a next generation clean energy source. However, the most common method for producing it—methane steam reforming—consumes large amounts of energy and releases significant greenhouse gas emissions. Photocatalytic hydrogen production, which relies on sunlight, is a cleaner alternative but faces challenges in maintaining stability under strong light and chemical stress. To overcome these limitations, the IBS research team introduced a strategy that stabilizes the catalyst within a polymer network while placing the reaction site at the interface between air and water. This setup allows the system to avoid common problems such as catalyst loss, poor gas separation, and reverse reactions. The system breaks down plastics like PET into useful byproducts such as ethylene glycol and terephthalic acid, while releasing clean hydrogen into the air. “The key was engineering a structure that works not only in theory but also under practical outdoor conditions,” explained Dr. LEE Wanghee, a postdoctoral researcher at MIT and co-first author of the study. “Every detail — from material design to the water-air interface — had to be optimized for real-life usability.” The researchers demonstrated that their system remained stable for over two months, even in highly alkaline conditions. The floatable catalyst system also works in diverse real-world water environments, including seawater and tap water In tests using a one-square-meter device placed outdoors under natural sunlight, the system successfully produced hydrogen from dissolved PET bottle waste. Additional economic and scale-up simulations showed that the technology can be expanded to 10 or even 100 square meters, offering a pathway toward cost-effective, carbon-free hydrogen production. “This research opens a new path where plastic waste becomes a valuable energy source,” said Professor KIM Dae-Hyeong. “It’s a meaningful step that tackles both environmental pollution and clean energy demand.” Professor HYEON Taeghwan added, “This work is a rare example of a photocatalytic system that functions reliably in the real world — not just the lab. It could become a key stepping stone toward a hydrogen-powered, carbon-neutral society.”
Notes for editors
- References
- Media Contact
- About the Institute for Basic Science (IBS) |
| Next | |
|---|---|
| before |
- Content Manager
- Public Relations Team : Yim Ji Yeob 042-878-8173
- Last Update 2023-11-28 14:20